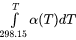PARAMETER
PARAMETER <parameter name> [lowest temp. limit]
[expression 1]; [upper temp. limit 1] Y
[expression 2]; [upper temp. limit 2] Y
[expression 3]; [upper temp. limit 2] Y
.......... ; ..... Y
[expression n-1]; [upper temp. limit n-1] Y
[expression n]; [upper temp. limit n] N {Ref. Index} !
This keyword can appear in both files for database definition and sequential storage, but not in FTP files. After the keyword, a valid < parameter name> in some cases should be given.
It is used to define standard Gibbs energies (i.e. the G parameters for Gibbs energy of formations) of all valid end-members of various stoichiometric and solution phases, and excess Gibbs energies (i.e. the L parameters for Gibbs energy of interactions) of all binary, ternary, quaternary or higher-order interactions in various solution phases; both standard Gibbs energies and excess energies can also have parameters for contributions from PT-depended volume variations (i.e. the V0, VA, VC and VK parameters for molar volume, thermal expansivity, bulk modulus, isothermal compressibility and high-pressure fitting parameter), magnetic ordering (i.e. the TC and BM parameters for Curie temperature and Bohr magneton number) and hypothetical electrostatic interactions (i.e. BM parameter for Born functions wPr,Tr of aqueous solute species).
The general form of a parameter is:
<identifier>(<phase>, <constituent array>; <digit>) <xxx> <expression> <yyy> <keyword Y or N> <zzz> !
| Name | Description |
|---|---|
|
identifier |
The parameter type. |
|
phase |
The phase name (maximum 24 characters). |
|
constituent array |
The specific constituent array in the phase. |
|
digit |
The degree of composition-dependent interaction contribution (an integer number from 0 to 9), that is only for excess energy (L), Curie temperature (TC) and Bohr magneton number (BMAGN), as well as for volume-related parameters (V0 or VA or VB or VC or VK); if it is valued as zero, or if it is for the standard Gibbs energy (G) for which the degree is always zero, it can be omitted. |
|
expression |
The mathematical relation to describe the parameter. |
|
xxx and yyy |
The low and high temperature limits respectively for the applicable temperature range of the parameter expression |
|
keyword Y or N |
The indicator on if there is continuation for the parameter expression or not |
|
zzz |
The reference index/number for the assessment of this parameter; |
|
! |
The exclamation mark is used to indicate that the current parameter definition is ended |
GIBBS Parameter Name
The GIBBS parameter name has a general form of:
<identifier>(<phase>,<constituent array>;<digit>)
The GIBBS parameter name consists of several parts. The first is a type-identifier. The following type-identifiers are legal:
| Type-Identifier | Definition |
|---|---|
|
G |
Standard energy parameter (Gibbs energy of formation) |
|
L |
Excess energy parameter (Gibbs energy of interaction) |
|
TC |
Curie temperature for magnetic ordering |
|
BMAGN or BM |
Bohr magneton number for magnetic ordering (or Born function |
|
LNTHETA1, LNTHETA2, LNTHETA3, LNTHETA4, and LNTHETA5 |
This parameter is only available with GES6. |
|
THETAF1, THETAF2, THETAF3, THETAF4, and THETAF5 |
A weight function used together with a corresponding LNTHETA parameter with the same number. Used by the general Einstein model. See General Einstein Model. This parameter is only available with GES6. |
|
V0 |
Molar volume at 298.15 K and 1 bar (a numeric value only) |
|
VA |
Integrated thermal expansivity |
|
VC |
High-pressure fitting parameter |
|
VK |
Isothermal compressibility |
|
VISC |
RT*ln(η) where η is the dynamic viscosity |
|
ELRS |
Electric resistivity |
|
THCD |
Thermal conductivity |
|
SIGM |
Surface tension of a liquid end-member |
|
XI |
Surface tension dampening factor/interaction for one constituent in a two-constituent combination. See the examples for how to use these parameters |
| ECij |
Elastic constant parameter |
| ECijMAG |
Scaling factor for magnetic contribution to elastic constants |
| Parameter | Definition |
|---|---|
G(GAS,C1O2)
|
The Gibbs energy of formation of a CO2 molecule in gas. |
G(FCC,FE:VA)
|
The Gibbs energy of formation of fcc Fe with interstitials. |
L(LIQ,Fe,Cr;0)
|
The regular solution parameter for Fe and Cr in liquid. |
L(LIQ,Fe,Cr;1)
|
The sub-regular solution parameter. |
TC(BCC,Fe:Va)
|
The Curie temperature of bcc Fe. |
BMAGN(BCC,Fe:Va)
|
The Bohr magneton number parameter of bcc Fe. |
|
|
Surface tension of Al in LIQUID. Constant value or linear temperature dependence. |
|
|
Surface tension dampening factor for Al in the Al-Cu combination in LIQUID. Constant value. |
|
|
Surface tension dampening factor for Cu in the Al-Cu combination in LIQUID. Constant value. |
EC11(HCP_A3, TI:VA;0)
|
The regular solution parameter for elastic constant C11 of HCP Ti with interstitials. |
EC11MAG(BCC_A2, FE:VA; 0)
|
The scaling factor for the magnetic contribution to elastic constant C11 for BCC Fe with interstitials. |
You can also use G for interaction parameters; and on output list (performed by the GIBBS command LIST_PARAMETER or LIST_PHASE_DATA) the type-identifier L is always used for interaction parameters. Note that the type-identifier BM is also used for Born functions wPr,Tr of aqueous solute species.
The identifier must be followed by an opening parenthesis, a phase name, a comma and a constituent array. Optionally, the constituent array can be followed by a semicolon and a digit. The parameter name is terminated by a closing parenthesis.
It is important that if a phase bears a legal phase-type (among G, A, Y, L, I, F and B) in its phase definition (already by the PHASE keyword; such as GAS:G, LIQUID:L, SLAG:L, IONIC_LIQ:Y, SPINEL:I, FCC_L12:F, HCP_D021:F, BCC_B2:B, AQUEOUS:A), such a valid phase-type code should not be attached to the phase name in the PARAMETER keyword.
Specifying the phase name in UPPER-case is recommended. You can define a phase name as a mixture of UPPER-case and lower-case letters in a database, but the DATA module automatically converts all lower-case to UPPER-case because the GIBBS module only recognizes UPPER-case phase names.
The constituent array consists of a list of constituent names. Interaction parameters have two or more constituents from the same sublattice separated by a comma. If the phase has sublattices, at least one constituent in each sublattice must be specified. The exception is if the phase is ionic liquid (specified with the "phase bit" :Y), then neutral endmember and interaction parameters are written as if the first sublattice does not exist, e.g. PARAM G(IONIC_LIQ,FEO3/2;0), i.e. the constituent array does not contain any constituents from the first (cation) sublattice.
For more ionic liquid specific parameters, see Parameters for the Two Sublattice Ionic Liquid Model.
The constituents in different sublattices must be given in sublattice order and are separated by a colon.
After the component array, a sub-index digit can be specified after a semicolon. This digit must be in the range 0 to 9. The interpretation of the sub-index depends on the excess energy model used for the phase. If no semicolon and digit are given, the sub-index value is assumed to be as zero.
The excess energy parameters, e.g. the regular/subregular (binary) parameter or ternary parameters, are multiplied with two or more fractions of the constituents from the same sublattice of the solution phase. These additional constituents must be given as interacting constituents.
Be careful about the sign of odd terms, for example, L(BCC, B, A:VA;1) is treated as L(BCC,A,B:VA;1), i.e. it is always put into alphabetical order.
Solution phases with sublattices may have interacting constituents in each sublattice.
You can use an asterisk (*) to denote that the excess interaction parameter is independent of the constituents of a specific sublattice. For example, L(FCC_L12,AL,NI:*) means that the interaction parameter is for the binary interaction between constituents AL and NI on the first sublattice in the FCC_L12 solution phase, while it is independent of all constituents on the second sublattice. A interaction parameter in the list of constituents is always added to the Gibbs energy and the asterisk (*) is calculated with the term of [1-∑y(specified constituents)], which implies that in an A-B binary system the following three L parameters are identical (but in higher-order systems, they are different):
L(phase,A,B)is multiplied withX(A)*X(B)
L(phase,A,*)is multiplied withX(A)*(1-X(A))
L(phase,B,*)is multiplied withX(B)*(1-X(B))
A parameter always starts with a lowest temperature limit of its applicability, followed by one or more (up to 10) expressions (TP-Functions) coded as mathematical relations of constants, functions of stable variables (T and P) and entered functions (normally with a # suffix, e.g. +3*GSERAL#).
The expression is a FORTRAN-like expression and operators +, -, *, = and ** can be used (** only with integer powers). Unary-functions LN or LOG (both for natural logarithm) and EXP (for exponential) can also be used.
There is also the Einstein function GEIN available:
GEIN(THETA) = 1.5*R*THETA + 3*R*T*LN(1-EXP(-THETA/T))
Each expression (TP-Function) should ends with a semicolon (;) and be followed by its upper applicable temperature limit and a continuation indicator (Y to continue with the next expression, or N to end the parameter’s expression). If there is no continuation after a specific expression (TP-Function), the reference index can be optionally given after the N indicator.
A complete/valid parameter entry can be written in several continuation lines if the parameter’s expression (TP-Function) is too long or if there is more than one applicable expression (TP-Function), as the maximum length of each line is 78 characters.
It is recommended to always have at least one empty space at the beginning of each continuation line. Avoid entering parameters such as:
PARAMETER G(LIQUID,A,B) 298.15
-2000+4568*T+2*GFUNAB#; 6000 N !
Such a parameter is read by the DATA module as 2000+4568*T+2*GFUNAB#, rather than as -2000 +4568*T+2*GFUNAB#.
Avoid this mistake by giving at least one empty space as the first character of a new line, such as
PARAMETER G(LIQUID,A,B) 298.15
-2000+4568*T+2*GFUNAB#; 6000 N !
The lowest-temperature limit (in Kelvin) for the applicability of the (first) TP-Function in a parameter is normally set by default as 298.15 K, in most cases; however, you can set another limit when it is applicable (according to experimental data and assessments). An upper-temperature limit (in Kelvin; followed by a Y or N sign) for the applicability of each TP-Function in a parameter must be given after the semicolon (;) immediately following the specific TP-Function; and the highest-temperature limit (in Kelvin) for the applicability of the current parameter is always followed by the N sign. If a negative number is given as the lowest-temperature limit, it is assumed that there are breakpoints in pressure for this parameter. In such cases, it is interpreted as the lowest-pressure limit (in Pascal), and the other limits in the current parameter are also taken as pressure limit values (in Pascal).
The temperature/pressure limits for the parameters are checked during calculations. An indicator is set if the actual temperature/pressure condition is below the lowest temperature/pressure limit or above the highest temperature/pressure limit. In these cases, an extrapolation is done using the TP-Function valid in the nearest temperature/pressure range.
The optional reference index {Ref. Index} is an integer number indicating where to find the particular parameter in a special reference file. The references are listed when doing the GET_DATA command in the DATA module. These can also be listed in the GIBBS module with the command LIST_DATA and the option R or N.
For accounting the reference indices, also see the keyword REFERENCE_FILE.
The reference index field can also be an abbreviation (such as REF:250, REF_002, or REF-SGTE) denoting the original reference. In this case, the reference cannot be obtained when issuing the DATA command GET_DATA or the GIBBS command LIST_DATA (with the option R or N).
However, the references directly coded in the database definition file (***setup.TDB) that starts with a letter can be shown when issuing the DATA command GET_DATA or the GIBBS command LIST_DATA (with the option R or N). Normally, such references must be located after the LIST_OF_REFERENCE keyword. It is recommended to use reference code names such as REF001, REF018, etc. The reference list, which is generated by the GIBBS command LIST_DATA <file> with the N option, is thus also possible to be directly read by the DATA module.
PARAMETER G(BCC,FE:VA) 298.15 1000+200*T+...; 6000 N 91DIN !
PARAMETER TC(BCC,FE:VA) 298.15 +1043; 6000 N 91DIN !
PARAMETER BMAGN(BCC,FE:VA) 298.15 +2.22; 6000 N 91DIN !
PARAMETER G(SIGMA,FE:CR:CR;0) 298.15 1000+200*T+...; 6000 N 101 !
PARAMETER G(LIQUID,AL;0) 298.15 +11005.553-11.840873*T
+7.9401E-20*T**7+GHSERAL#;
933.60 Y +10481.974-11.252014*T+1.234264E+28*T**(-9)+GHSERAL#;
2900.00 N REF:283 !
PARAMETER G(BCC_A2,PB:C) 298.15 UN_ASS#; 300 N REF:0 !
PARAMETER G(BCC_A2,NI:C;0) 298.15 +GHSERNI#+3*GHSERCC#
+400000-100*T; 6000 N REF071 !
PARAMETER G(BCC_A2,MN:VA) 298.15 +GMNBCC#; 6000 N REF285 !
PARAMETER G(PHASE,A:B) 298.15 +3*GEIN(800); 6000 N 101 !
PARAMETER BM(AQUEOUS,OH-1) 298.15 +Z0002PW0#; 1600 N 155 !
PARAMETER L(BCC,FE,CO:VA;0) 298.15 1000+200*T+...; 6000 N !
PARAMETER L(BCC,FE,CO:VA;1) 298.15 1000+200*T+...; 6000 N !
PARAMETER L(BCC,FE,CO:VA;2) 298.15 1000+200*T+...; 6000 N !
PARAM TC(BCC_A2,CO,MO:VA;0) 298.15 -3700; 6000 N R454 !
PARAM TC(BCC_A2,CO,MO:VA;1) 298.15 +2300; 6000 N R454 !
PARAM BMAGN(BCC_A2,CO,MO:VA;0) 298.15 -3.445; 6000 N R454 !
PARAM V0(BCC_A2,CR,FE:VA;0) 298.15 +ZERO#; 6000 N REF06V !
PARAM V0(BCC_A2,CR,FE:VA;1) 298.15 -1.10524097E-7; 6000 N REF06V !
PARAM V0(BCC_A2,CR,FE:VA;2) 298.15 +1.40024130E-7; 6000 N REF06V !
PARAM VA(BCC_A2,CR,FE:VA;0) 298.15 -6.49444634E-6*DELTAT#; 6000 N REF06V !
PARAM VA(BCC_A2,CR,FE:VA;1) 298.15 +2.91269321E-5*DELTAT#; 6000 N REF06V !
Parameters for the Two Sublattice Ionic Liquid Model
For the two sublattice ionic liquid model (specified with the "phase bit" :Y in the phase declaration), there are some additional requirements on the allowed parameters. While in the regular case all combinations of a phase’s constituents are allowed as end-members, there are some constraints on the allowed parameters for ionic liquid.
In the ionic liquid model, the vacancy has been given an “induced” charge -Q, where Q is the average charge of the first (cation) sublattice.
All allowed parameters are listed below. The following notations are used:
C= CationC1= Cation 1 if more than one. Similar for anions and neutrals.A= AnionVA= VacancyN= NeutralD= Anion or vacancy or neutral (i.e. anything on the second sublattice)
The phase description is:
(C1,C2…Ci)P(A1,A2...Ai,VA,N1, N2...Ni)Q
In the ionic liquid model,the Vacancy has been given an “induced” charge -Q, where Q is the average charge of the first (cation) sublattice.
Allowed end-members, assuming the phase name is IONIC_LIQ:
G(IONIC_LIQ,C:A;0)
G(IONIC_LIQ,C:VA;0)
G(IONIC_LIQ,N;0)
(The last line is an exception to the case that the constituent array must contain constituents from all sublattices)
This leaves one not allowed end-member:
G(IONIC_LIQ,C:N;0)
Allowed binary interaction parameters, where i is the interaction order in a Redlich-Kister expansion.
G(IONIC_LIQ,C1,C2:A;i)
G(IONIC_LIQ,C1,C2:VA;i)
G(IONIC_LIQ,C:VA,N;i)
G(IONIC_LIQ,N1,N2;i)
G(IONIC_LIQ,C:A1,A2;i)
G(IONIC_LIQ,C:A,VA;i)
G(IONIC_LIQ,C:A,N;i)
No other binary interactions are allowed.
Allowed ternary interaction parameters. The interaction is composition independent if there is one parameter with i = 0. It is composition dependent if there are three parameters with i = 0, 1 and 2. There can only be one or three parameters for one combination of constituents
G(IONIC_LIQ,C1,C2,C3:A;i)
G(IONIC_LIQ,C1,C2,C3:VA;i)
G(IONIC_LIQ,N1,N2,N3;i)
G(IONIC_LIQ,C1,C2:VA,N;i)
G(IONIC_LIQ,C:VA,N1,N2;i)
G(IONIC_LIQ,C:A,D1,D2;i)
G(IONIC_LIQ,C1,C2:A,D;i)
No other ternary interactions are allowed.
 Pr,Tr for aqueous solute species).
Pr,Tr for aqueous solute species).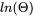 where
where  is the Einstein temperature. Used by the general Einstein model and together with a corresponding THETAF parameter with the same number. See
is the Einstein temperature. Used by the general Einstein model and together with a corresponding THETAF parameter with the same number. See