Including or Excluding the Gas Phase
Under defined pressure and temperature conditions, the solvent water’s thermodynamic stability limits are determined by the following two electrochemical reactions:
H2O(water) + 2e- = H2(gas) + 2OH-1
2H2O(water) = O2(gas) + 4H+1 + 4e-
The first reaction describes the formation of H2-dominated gaseous mixture, under reducing conditions. The second reaction describes the formation of O2-dominated gaseous mixture, under oxidising conditions.
If the system reaches global equilibrium, then the water component is electrolyzed to H+ and O-2 at all pH conditions. The degree of electrolysis depends on the pH value in the aqueous solution phase. If Eh gets high enough, then the O-2 anion is oxidized to O2 (aqs). On the other hand, if Eh gets low enough, then the H+ cation is reduced to H2 (aqs). The major electrolysis and redox reactions are the following:
H2O (water) = H+ + OH- → Electrolysis of water at all pH.
H2O (water) = 2H+ + O-2 → Electrolysis of water at all pH.
O-2 - 2e- = 0.5O2 (aqs) → Oxidation or de-electronization of O-2 at high Eh.
2H+ + 2e- = H2 (aqs) → Reduction or electronization of H+ at low Eh.
At a critically high Eh value under a given pH condition, an aqueous solution phase with a high enough O2 activity becomes less stable than an O2-dominated gaseous mixture phase. At this point, the gas phase replaces the aqueous solution phase in the system. The replacement process can be characterised by the following phase-transformation on the aqueous-gas phase boundary and oxidation of remaining water:
O2 (aqs) = O2 (gas)
H2O (water) - 2e- = 2H+(gas) + 0.5O2 (gas)
Similarly, at a critically low Eh value under a given pH condition, an aqueous solution phase with a high enough H2 activity becomes less stable than a H2-dominated gaseous mixture phase. At this point, the gas phase replaces the aqueous solution phase in the system, through the following phase-transformation on the aqueous-gas phase boundary and reduction of remaining water:
H2 (aqs) = H2 (gas)
H2O (water) + 2e- = O-2 (gas) + H2 (gas)
The phase transformation from an aqueous solution phase to an O2- or H2-dominated gaseous mixture phase also depends on the total molar Gibbs energies of the phases (which are complex functions of the phase constituents, the temperature and the pressure). The Gibbs energy minimization technique used in Thermo‑Calc ensures that the phase transformation is accurately simulated.
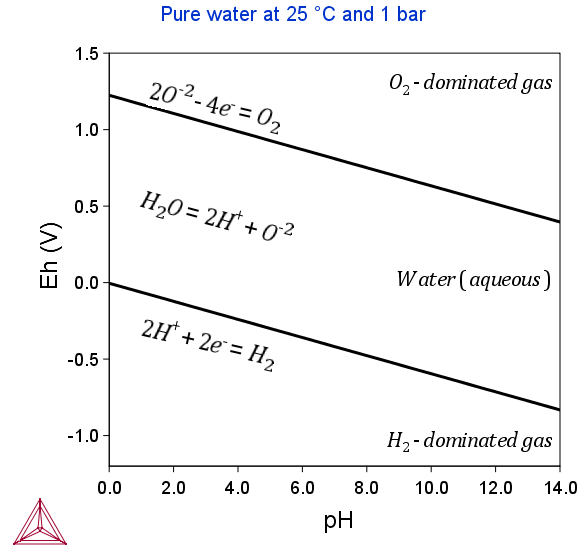
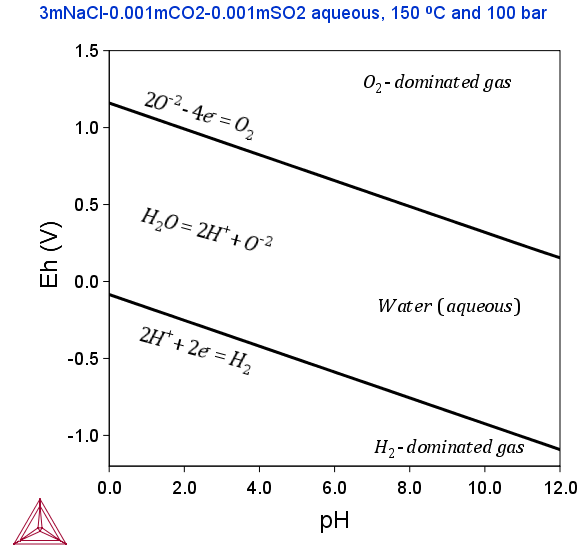
The following Pourbaix diagrams show the result of a calculation where the gaseous mixture phase was included. In the upper region (high Eh) and lower region (low Eh), the aqueous solution phase has been oxidized and reduced, respectively, to an O2- or H2-dominated gaseous mixture phase.
The two example diagrams show that the upper and lower boundaries between the aqueous phase (water) and the gas phase (dominated by either O2 or H2) can shift when solutes dissolve or when the temperature and pressure change.
Accordingly, if one does not take a gaseous mixture phase into account when performing a calculation of Pourbaix diagram (as above), then the aqueous solution phase may end up with an extremely high O2 (aqs) concentration at high Eh condition, or an extremely high H2 (aqs) concentration at low Eh condition. Under either of these two extreme circumstances, the concept of “aqueous solution phase” is no longer valid and consequently no proper aqueous solution model can actually be applied. Therefore, from a restrictive thermodynamic equilibrium point of view, one shall normally include a gaseous mixture phase in an aqueous-bearing heterogeneous interaction system. This is true for all types of equilibrium calculations for an aqueous-bearing heterogeneous interaction system, not only of Pourbaix diagram calculations.
Hence, to perform a completely accurate Pourbaix diagram calculation, the gaseous mixture phase must be taken into account. However, since these electrochemical reactions normally have much higher kinetic barriers and are slower than other electrochemical and chemical reactions in the interaction system, one can sometimes ignore the gaseous mixture phase in the calculation. Note that if the gaseous mixture phase is ignored in this way, then the Pourbaix diagram does not show the H2O-stability limiting lines.