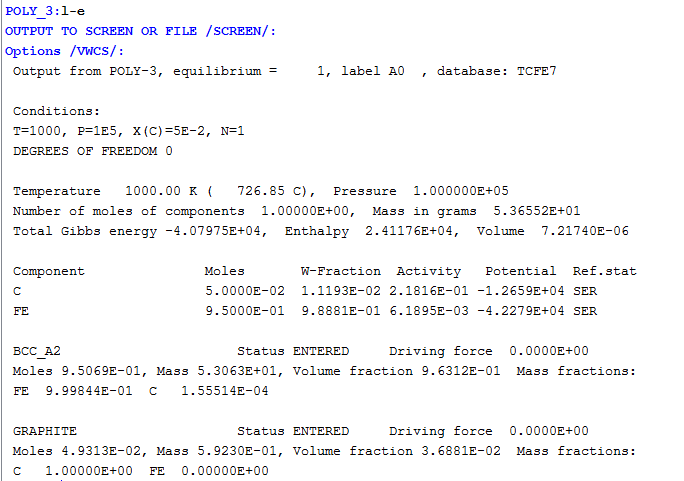
Setting Conditions for Equilibrium Calculations
Setting a condition normally involves giving a single state variable a specific value. For example, you can set the temperature to 1273.5 Kelvin (T=1273.5). Alternatively, setting a condition can involve giving a linear expression with more than one state variable a specific value. For example, you can set the mole fraction of the S component to be the same in the liquid and the pyrrohotite phases (X(LIQ,S)-X(PYRR,S)=0).
The number of state variables that need to set is determined by the Gibbs Phase Rule. Typically, the state variables to give values to are:
- temperature (in K)
- pressure (in Pascal)
- system size in number of moles (in mole) or mass (in grams)
- the fraction of each component (in number of moles or mass)
If you fix the phase of the equilibrium and all but one of the state variables, you can discover the value of the other state variable at equilibrium.
It is possible to specify a set of conditions that does not have any equilibrium and the program detects this by failing to reach equilibrium during the calculation.
The table lists some of the available state variables you can use to set conditions.
| State Variable | SET_CONDITION Parameter |
|---|---|
|
temperature in the system (in K) |
T |
|
pressure in the system (in Pascal) |
P |
|
system size (mole number in moles) |
N |
|
system size (mass in grams) |
B |
|
number of moles of a component in the system |
N(<component>) |
|
mole fraction of a component in the system |
X(<component>) |
|
mass fraction of a component in the system |
W(<component>) |
|
activity of a component in the system |
ACR(<component>) |
|
chemical potential of a component in the system |
MUR(<component>) |
|
mole fraction of a component in a phase |
X(<phase>,<component>) |
|
mass fraction of a component in a phase |
W(<phase>,<component>) |
|
activity of a species referred to a phase at ambient temperature and pressure |
ACR(<species>,<phase>) |
|
chemical potential of a species referred to a phase at ambient temperature and pressure |
MUR(<species>,<phase>) |
|
enthalpy in the system (in J) |
H |
|
enthalpy of a phase (in J/mol) |
HM(<phase>) |