About the Tabulation Reaction Module
The TABULATION_REACTION (TAB) module can be used to tabulate thermodynamic functions of any type of substance, stoichiometric phase or solution phase, or reaction. The module can tabulate thermodynamic functions of pure stoichiometric phases or solution phases at fixed composition, as well as various kinds of partial derivatives for a pure stoichiometric phase or solution phase at a given composition.
The TAB module begins by evaluating the most stable species of a substance in either a homogeneous state or in a reaction. Results are presented in a table or plotted as a graph.
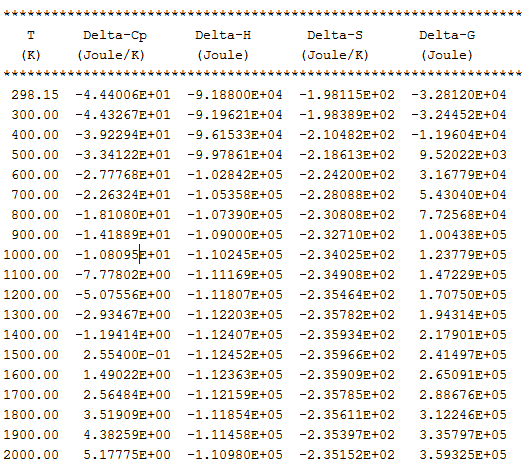
A reaction tabulation presents you with the following properties, on rows representing different temperature levels:
- Heat capacity change / Delta-Cp (Joule/K) in column 2.
- Enthalpy change / Delta-H (Joule) in column 3.
- Entropy change / Delta-S (Joule/K) in column 4.
- Gibbs energy change / Delta-G (Joule) in column 5.
In the case of a tabulation of a substance or solution phase at a fixed composition, the properties presented are the heat capacity, enthalpy, entropy and Gibbs energy, rather than the degree of change in those properties.
Tabulating a Substance or Solution Phase at Fixed Composition
You can add a 6th column to the table that shows the values of a property or user-defined function of your choice.