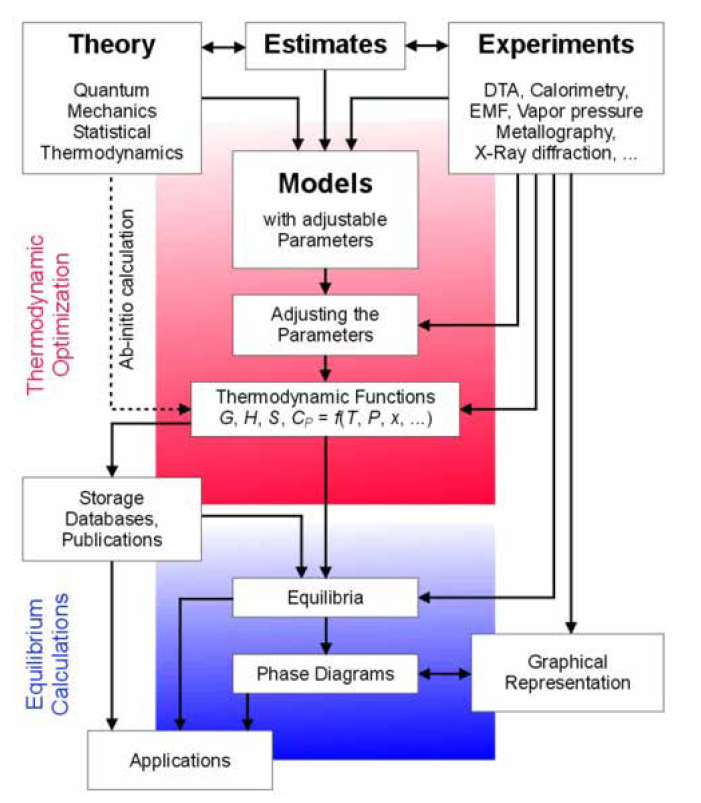
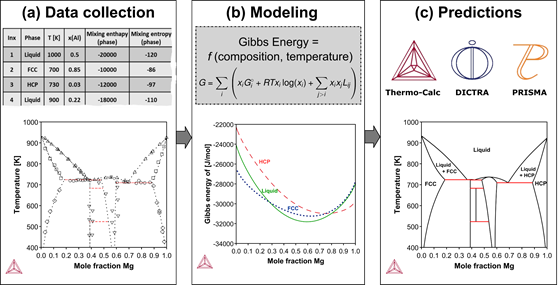
The CALPHAD Method and the Thermo‑Calc Databases
CALPHAD is originally an abbreviation for CALculation of PHAse Diagrams, but was later expanded to refer to computer coupling of phase diagrams and thermochemistry. More about the CALPHAD methodology, including some of its history, is available on the Thermo‑Calc Software website.
For more learning resources about CALPHAD and our databases, visit the video tutorials on our website or our YouTube playlist.
To make a calculation with Thermo‑Calc, it is necessary to select a database from which the thermodynamic data are obtained. These databases are developed using the CALPHAD (CALculation of Phase Diagrams) approach, which describes both the thermodynamics and phase equilibria of a system as a function of chemistry and temperature in a self-consistent framework. This approach enables the prediction of properties of multicomponent systems based on data obtained from the critical assessment of binary and ternary subsystems. These assessments are combined to construct a multicomponent database.
CALPHAD is a phase-based approach, whereby the thermodynamic properties of each phase are described through the Gibbs free energy, 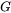 , which is a function of the chemical composition, temperature, and pressure of a material. The Gibbs free energy is evaluated through a critical assessment of all experimental and theoretical information available on phase equilibria and thermochemical properties in a system. Additionally, physical and chemical properties of the system such as crystallography, type of bonding, order-disorder transitions and magnetic properties are also considered.
, which is a function of the chemical composition, temperature, and pressure of a material. The Gibbs free energy is evaluated through a critical assessment of all experimental and theoretical information available on phase equilibria and thermochemical properties in a system. Additionally, physical and chemical properties of the system such as crystallography, type of bonding, order-disorder transitions and magnetic properties are also considered.
Building a CALPHAD Database
More information specific to Thermo‑Calc is contained in the Database Manager User Guide, which is part of the Thermo‑Calc Documentation Set PDF on the web, or search the Thermo‑Calc online help
The following is a basic overview about how the thermodynamic data contained within a CALPHAD database is collected and assessed. Within the CALPHAD methodology, for a given thermodynamic system with elements A and B, there exists a number of thermodynamically distinct regions or so-called phases. The materials science professionals who compile the information in these databases use their experience to assign a model for the Gibbs energy to each of these phases. The mathematical function used to model the Gibbs energy is a polynomial in composition and temperature. Depending on the structure of each phase, various terms appear in the polynomial function and these terms are related to thermochemical properties of the system, e.g., formation enthalpy, configurational entropy, heat capacity, etc.
After assigning the models to these phases, the experts fit the free parameters of the model to the experimental data. At different stages of the assessment, this process requires extensive human judgment, often because the free parameters of all phases should be consistent with each other. In other words, the modeling task is a multi-objective optimization with constraints. Once the parameters for all phases are fitted to the experimental data, the optimized Gibbs energy models are collected in a text file (a so-called TDB file) with a format readable by the Thermo‑Calc calculation engine. These are the database files included with any Thermo‑Calc software installation.
The standard CALPHAD modeling steps are data collection, modeling, and prediction:
- Data collection: The first step is to collect data for materials systems and phases. The data is primarily from experimental and high-fidelity simulation calculations.
- Modeling: A materials scientist expert in the CALPHAD method then defines a hypothesis space for the mathematical expression of the model, a process in which they rely on experience and intuition. Using a trial-and-error approach, they then assess several of the hypothetical expressions by fitting its free parameters to the data until one is found that satisfactorily describes the target system.
- Predictions: Finally, the models are loaded into the Thermo‑Calc software to calculate thermodynamic properties of the systems of interest.
Accuracy and Validation
The accuracy of the calculations using Thermo‑Calc depends on the quality and completeness of the database used. In the case of the solution databases, generally the more binary, ternary and high order systems that have been assessed, the more wide-ranging the composition space will be and the more accurate the predictions. This information, along with examples of validation of the databases, are available on our website. Every effort is made to validate the databases as broadly as possible. However, since the CALPHAD approach allows for predictions to be made for multicomponent systems of any composition, critical calculations should always be verified by experimental data.
Scientific Bibliography
See the Thermo‑Calc Software scientific bibliography on our website.