Isothermal and Vertical Sections
Included ternary systems in the TCS Noble Metal Alloys Database (TCNOBL) are critically assessed based on available experimental and theoretical data for phase diagram and thermodynamic properties.
A variety of ternary systems are useful for alloy development with the database. Ternary and binary systems are the building blocks of a CALPHAD assessment.
Typical ternary phase diagrams are isothermal and vertical sections. Such diagrams can be of practical applications as well, e.g. making a preliminary determination of the heating temperature for melting, solution treatment, homogenization, and aging for specific alloys.
Calculated phase diagrams of some important ternary systems for noble metal alloys are shown, i.e. Ag-Cu-Zn, Ag-Au-Zn, Au-Cu-Zn, Ag-Au-In, Ag-Au-Cu, Ag-Cu-Ni, Au-Cu-In, Au-Ni-Pt, and Pd-Rh-Ru.
When working in Thermo‑Calc with ternary diagrams you use either the Ternary Calculator (in Graphical Mode) or the Ternary module (in Console Mode). The fundamental calculation engine is the same but you access the settings in different ways.
Ag-Cu-Zn
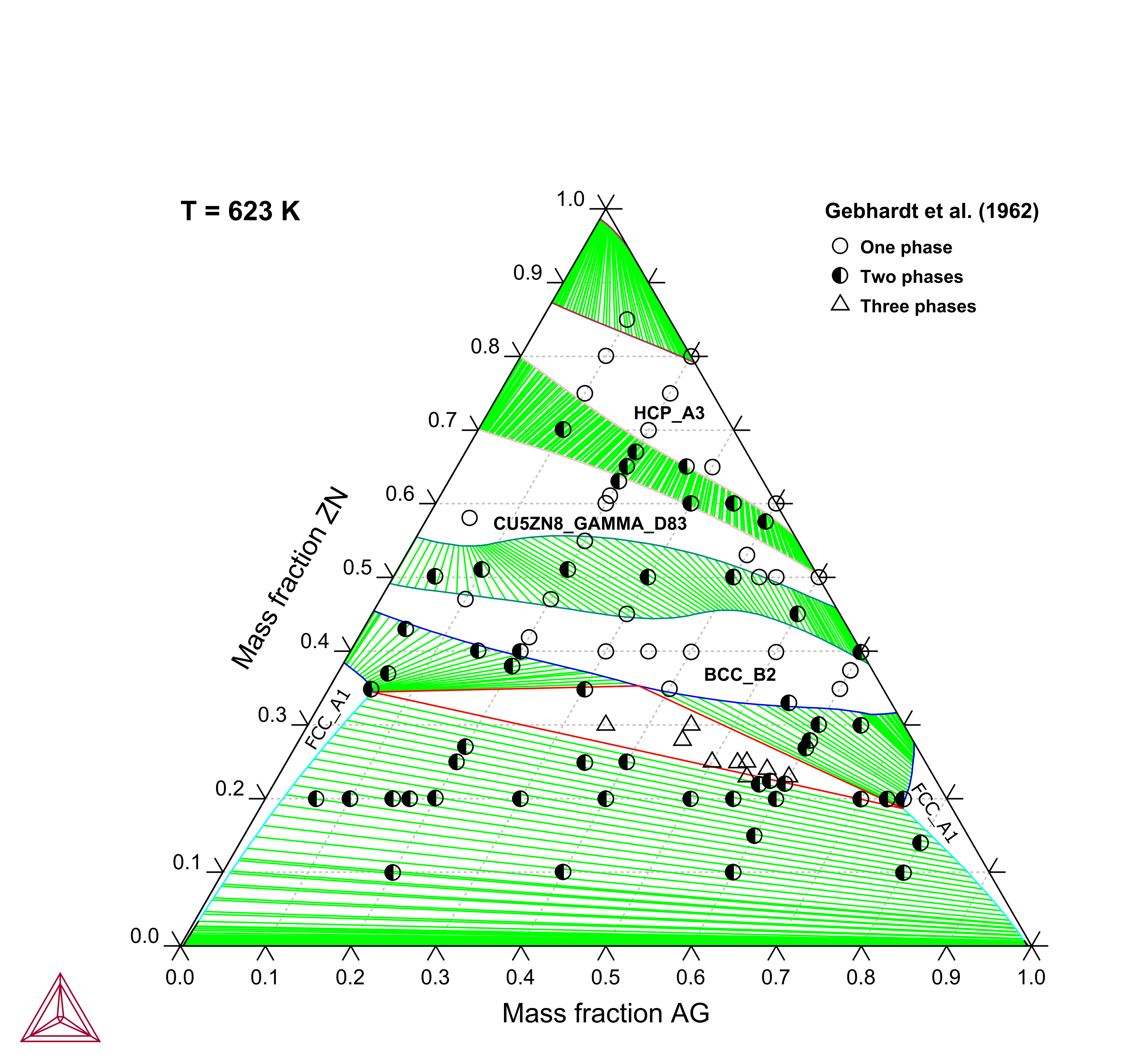
Figure 1: Calculated Ag-Cu-Zn isothermal section at 623 K compared with experimental data [1962Geb].
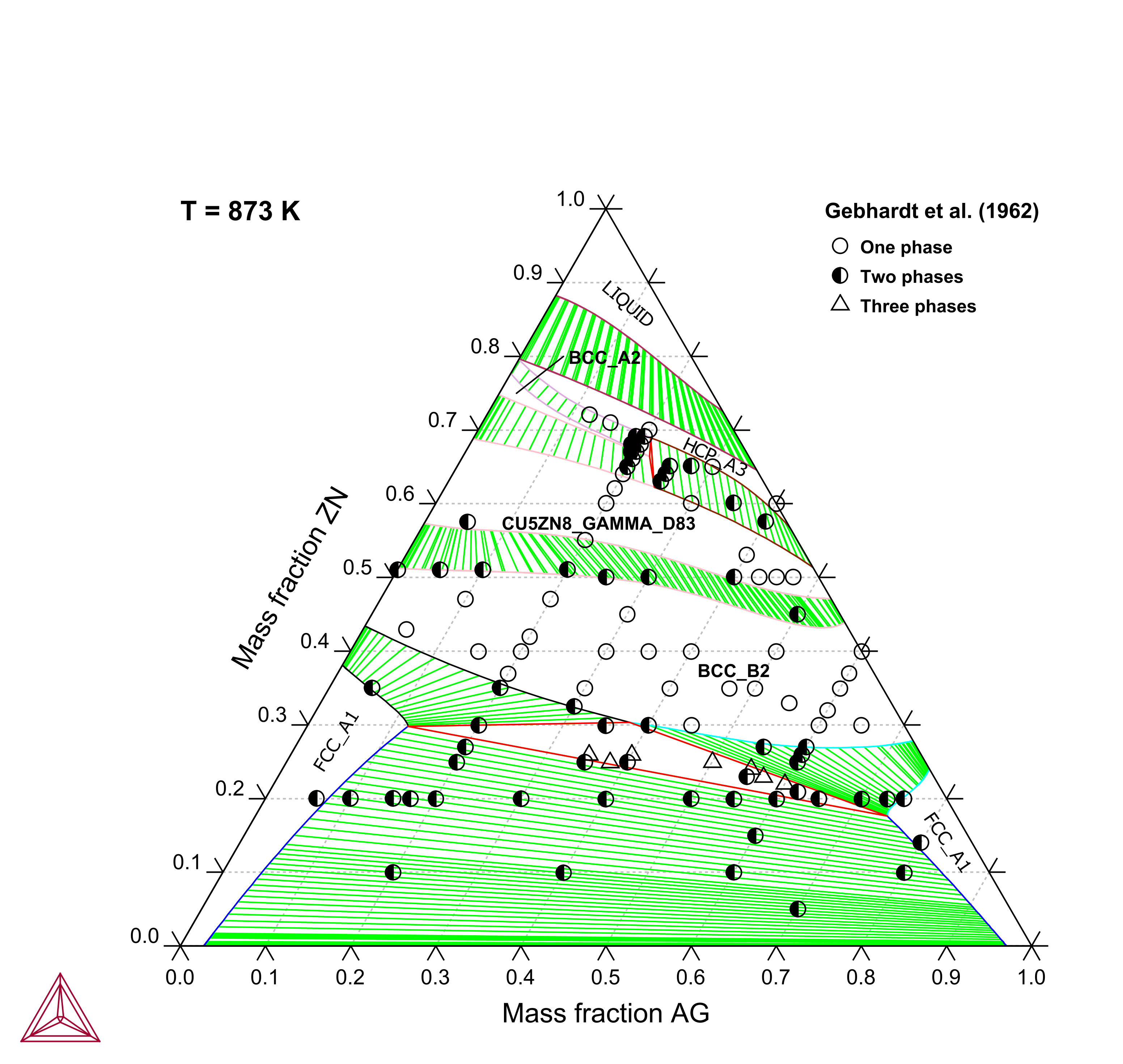
Figure 2: Calculated Ag-Cu-Zn isothermal section at 873 K compared with experimental data [1962Geb].
Ag-Au-Zn
Au-Cu-Zn
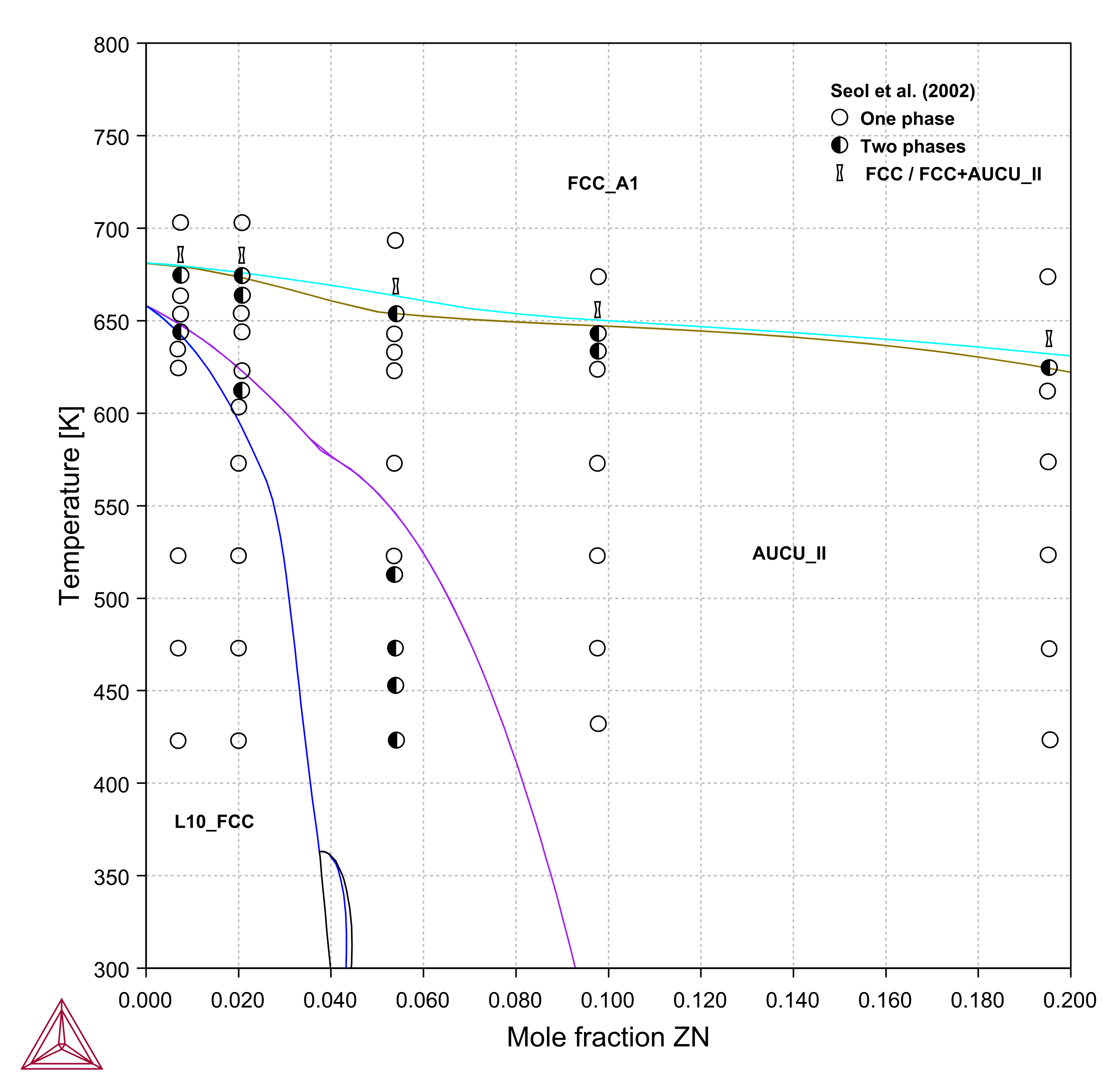
Figure 4: Calculated partial phase diagram for the AuCu-Zn pseudobinary system (up to 20 at.% Zn) compared with experimental data [2002Seo].
Ag-Au-In
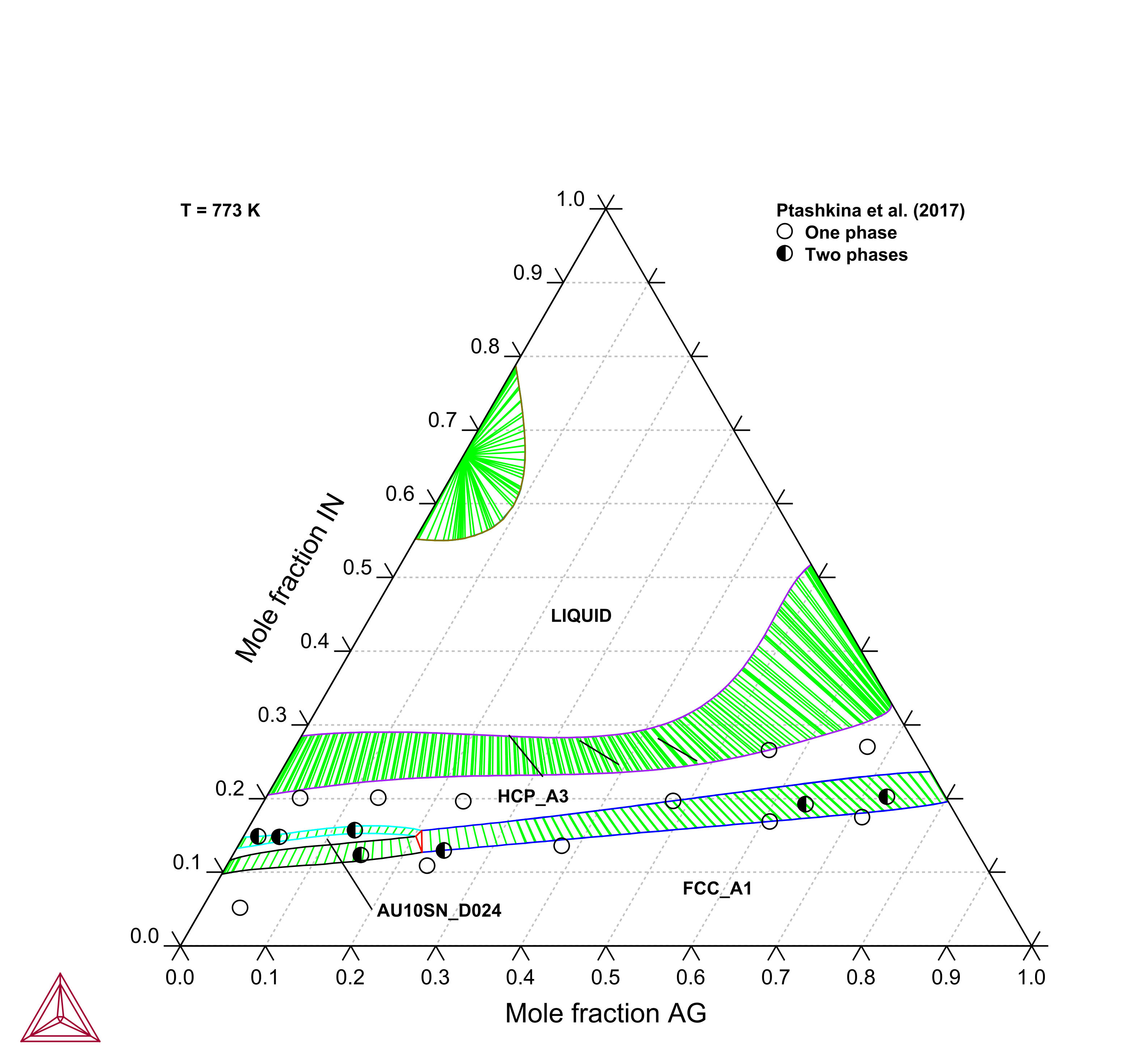
Figure 5: Calculated Ag-Au-In isothermal section at T=773 K compared with experimental data [2017Pta].
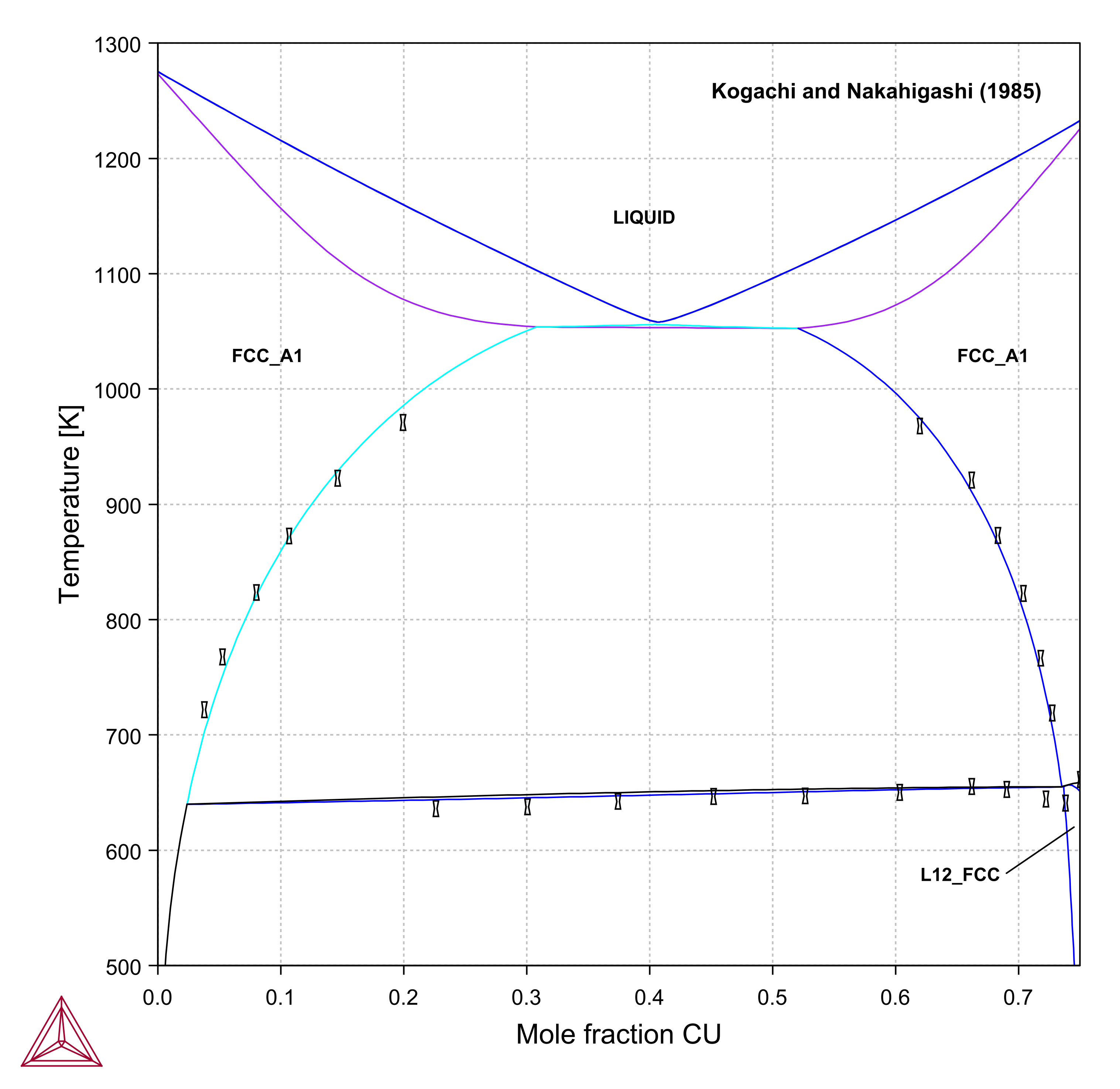
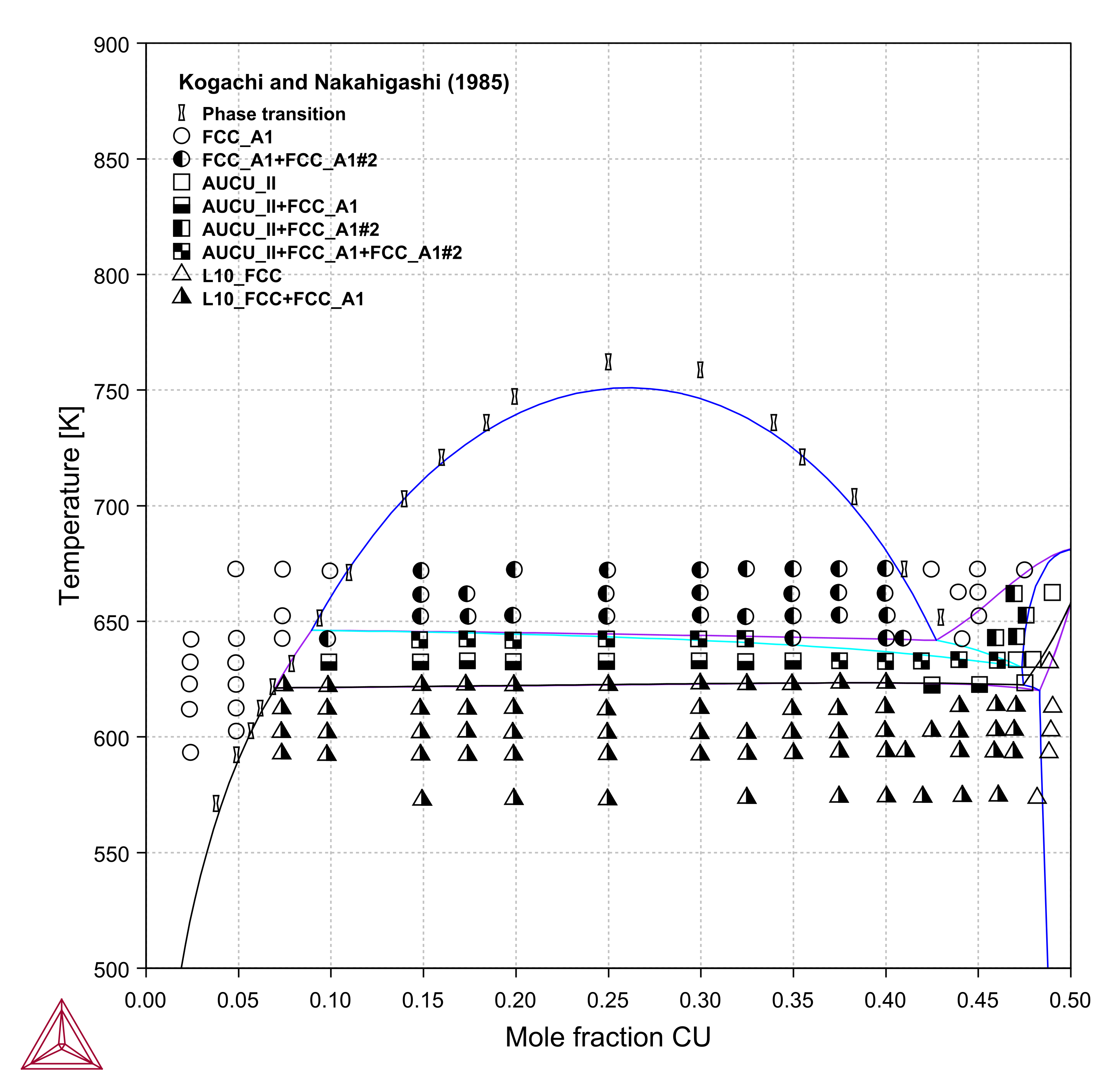
Ag-Au-Cu
|
|
Figure 6: Calculated Ag-Au-Cu vertical section at (left) 25 at. % Au, and (right) 50 at. % Au compared with experimental data [1985Kog].
Ag-Cu-Ni

|

|
Figure 7: Calculated Ag-Cu-Ni isothermal sections at (left) 1250 °C, and (right) 1400 °C compared with experimental data [1933Gue].
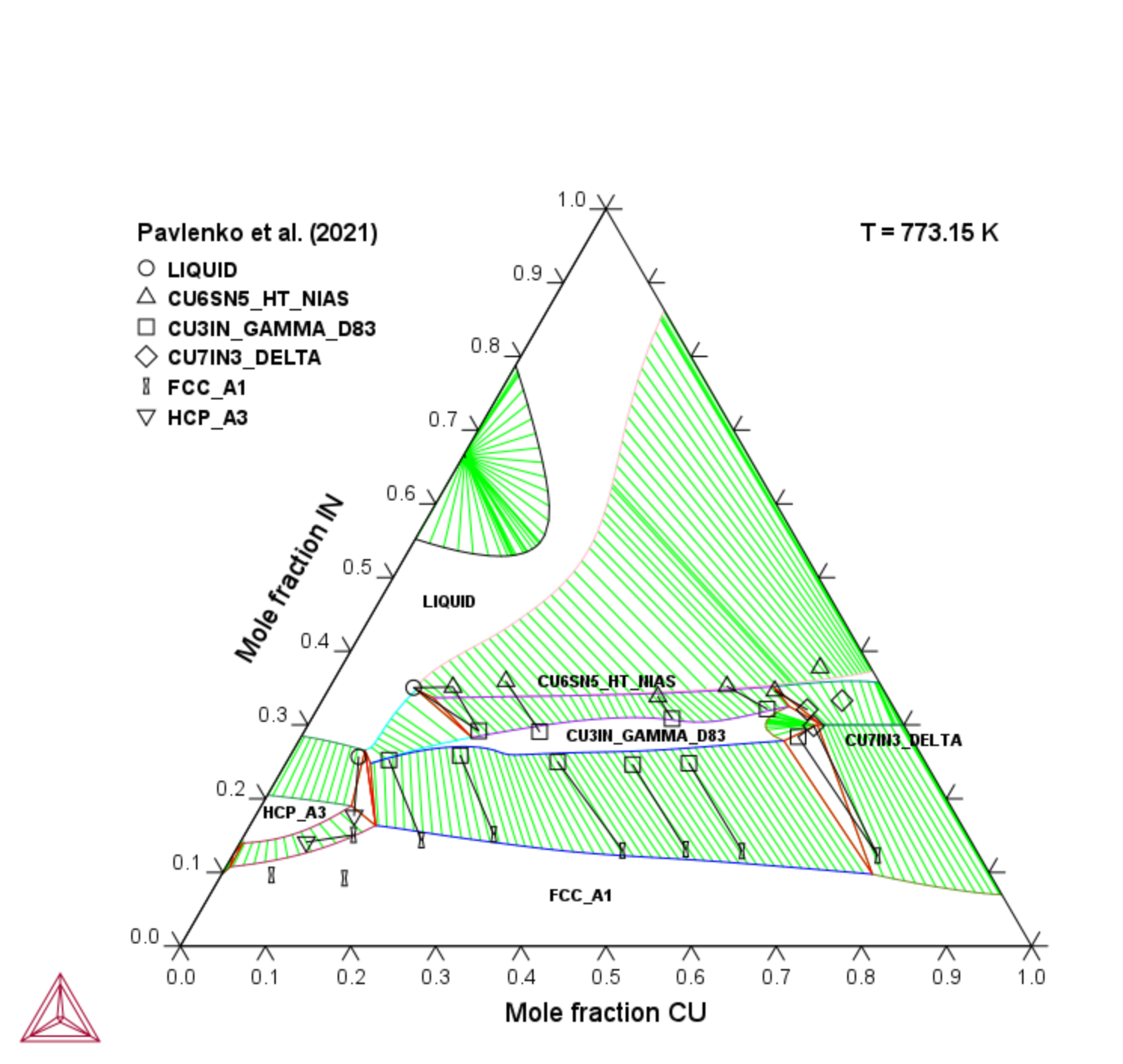
Au-Cu-In
Figure 8: Calculated Au-Cu-In isothermal section at 773.15 K compared with experimental data [2021Pav].
Au-Ni-Pt

|

|
Figure 9: Calculated Au-Ni-Pt isothermal sections at (left) 950 °C, and (right) 1150 °C compared with experimental data [1973Car].
Pd-Rh-Ru
Figure 10: Calculated Pd-Rh-Ru isothermal section at 1400 °C compared with experimental data [1984Rae].
References
[1933Gue] W. Guertler, A. Bergmann, Studies of the Ternary Silver-Copper-Nickel System. Zeitschrift für Met. 25, 53 (1933).
[1962Geb] E. Gebhardt, G. Petzow, W. Krauss, On the Constitution of the Cu-Ag-Zn System. Zeitschrift für Met. 53, 372–379 (1962).
[1969Bro] M. E. Brookes, R. W. Smith, Long range ordering in the system AgAuZn. Scr. Metall. 3, 667–669 (1969).
[1980Mat] Y. Matsuo, The Effect of Additional Elements on the βʹ → ϵ Transformation in Equiatomic AgZn Alloy. Trans. Japan Inst. Met. 21, 174–178 (1980).
[1951Mul] L. Muldawer, X‐Ray Measurement of Long‐Range Order in β‐AgZn. J. Appl. Phys. 22, 663–665 (1951).
[1957Liu] Y.-H. LIU, C.-C. HSU, An Investigation of Ag-Au-Zn Alloys with 50 AT. % Zn. Acta Phys. Sin. 13, 463–482 (1957).
[1973Car] S.M. Carmio and J.L. Merijering, “The Gold-Nickel- Platinum System,” Z.Metall, vol. 64, pp. 170-175, 1973.
[1984Rae] M.V. Raevskaya, V.V. Vasekin, I.G. Sokolova, “The interaction of platinum metals at 1400 °C,” J. Less-Common Met., vol. 99, pp. 137-142, 1984.
[1985Kog] M. Kogachi and K. Nakahigashi, “Phase relations in the AuCu1-yAgy and Au(Cu1-yAgy)3 ternary system,” Jpn. J. Appl. Phys., vol. 24, pp. 121-125, 1985.
[2002Seo] H.-J. Seol, T. Shiraishi, Y. Tanaka, E. Miura, Y. Takuma, K. Hisatsune, Partial phase diagram for the AuCu–Zn pseudobinary system. J. Alloys Compd. 339, 144–148 (2002).
[2017Pta] E. A. Ptashkina, A. G. Romanova, A. S. Pavlenko, E. G. Kabanova, V. N. Kuznetsov, Phase equilibria in the Ag–Au–In system at 500°C. Russ. J. Phys. Chem. A. 91, 264–267 (2017).
[2021Pav] A. S. Pavlenko, E. A. Ptashkina, G. P. Zhmurko, S. E. Philippova, E. G. Kabanova, V. N. Kuznetsov, Phase equilibria in the Au–Cu–In ternary at 500 °C: Experimental study and CALPHAD modeling. Calphad. 72, 102236 (2021).