Transition Temperatures
The TCS Ti/TiAl-based Alloys Database (TCTI) can be used to analyze α- and β-transus temperatures as shown in these examples.
α-transus Temperature
The α-transus temperature is an important parameter to develop processing techniques that are essential to improve mechanical properties of γ-TiAl-based alloys.
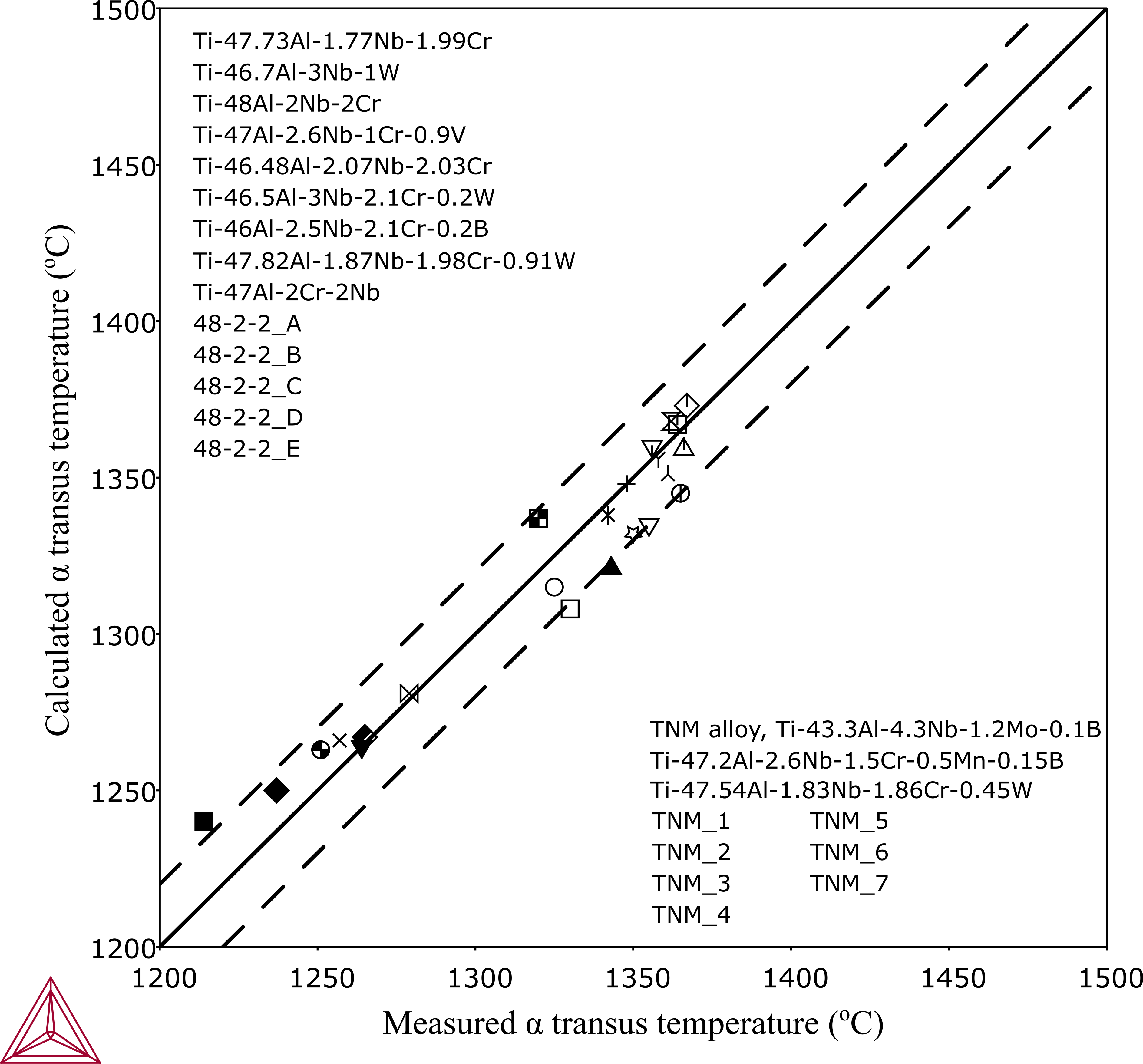
Validations have been focused on α-transus temperatures in the development of the thermodynamic database. One example shows a comparison between calculated and measured α-transus temperatures in typical multicomponent alloys.
The reported α-transus temperatures—measured either from thermal analysis or with synchrotron diffractions—can be affected by the deviation from equilibrium conditions.
Figure 1: A comparison between measured and calculated α-transus temperatures compared to experimental data. The dash lines mark for ±20 °C deviations.
β-transus Temperature
Beta (β) transus temperature (Tβ) is one of the important characteristics of titanium alloys. It is usually taken as the reference point to design a thermomechanical treatment. Therefore, knowledge of β-transus temperature is of critical importance to alloy design.
An important aspect for the development of the TCS Ti/TiAl-based Alloys Database (TCTI) is the validation of Tβ for industrial alloys. With a well-determined composition for an alloy including impurities, it is feasible to predict Tβ by performing a single point equilibrium type of calculation.
The following table is an example on the stable predictions of Tβ from this thermodynamic database for variant Ti-64 investigated in references.
|
Ti-64 Composition (wt%, impurities in wt. ppm) |
Reference | Tβ(exp.), °C | Tβ(calc.), °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | V | O | Fe | C | N | H | |||
| 6.1 | 4.1 | 2400 | 300 | 200 | 100 | - | [1991Lee] | 1005 | 994 |
| 6.04 | 4.03 | 900 | 1200 | 300 | 90 | 23 | [1998Ahm] | 994 | 989 |
| 6.4 | 4.2 | 1900 | 1400 | 160 | 50 | 40 | [2003Sem] | 1000 | 991 |
| 6 | 4.2 | 1100 | 1700 | 140 | 90 | 28 | [2005Elm] | 975 | 981 |
| 6.16 | 3.98 | 1900 | 1500 | 150 | 70 | - | [2014Sab] | 995 | 991 |
| 6.33 | 4.07 | 1600 | 1900 | 100 | 100 | 48 | [2019Sem] | 988 | 987 |
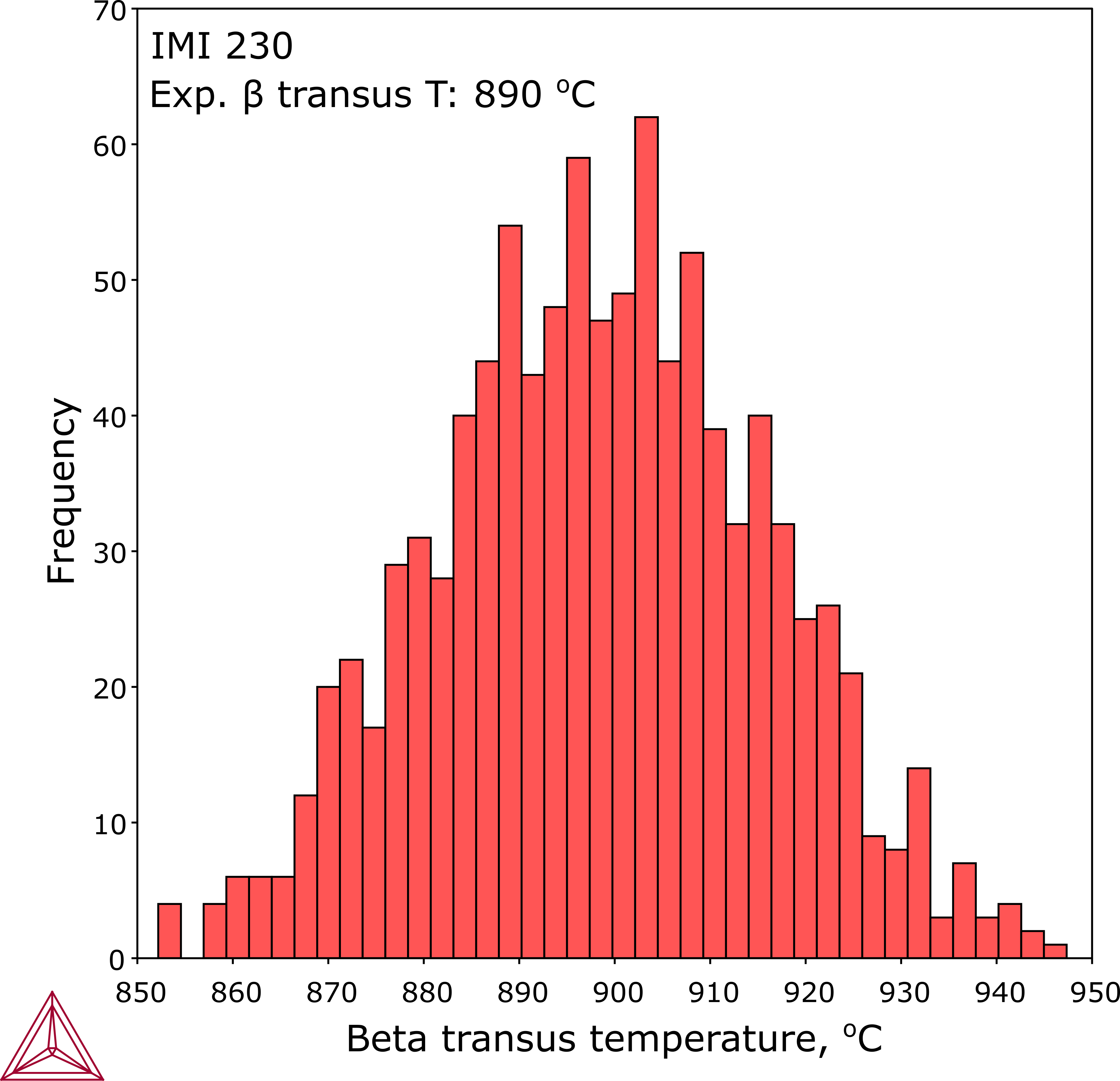
In practice, designed alloys typically have a nominal or target chemistry and an allowable tolerance range for each element. Determining the sensitivity of Tβ can be achieved for all combinations of chemistry within the allowed range, thus establishing a range for Tβ rather than a single value based on nominal composition. This example is for an IMI 230 alloy, which presents the variation of Tβ over the normal composition range in the corresponding table.
| Element in IMI 230 | Minimum Wt% | Maximum Wt% |
|---|---|---|
| Copper (Cu) | 2.00 | 3.00 |
| Iron (Fe) | - | 0.20 |
| Oxygen (O) | - | 0.20 |
| Carbon (C) | - | 0.08 |
| Nitrogen (N) | - | 0.03 |
| Hydrogen (H) | 0.01 |
References
[1991Lee] Y. T. Lee, M. Peters, G. Welsch, Elastic moduli and tensile and physical properties of heat-treated and quenched powder metallurgical Ti-6Al-4V alloy. Metall. Trans. A. 22, 709–714 (1991).
[1998Ahm] T. Ahmed, H. J. Rack, Phase transformations during cooling in α+β titanium alloys. Mater. Sci. Eng. A. 243, 206–211 (1998).
[2003Sem] S. L. Semiatin, S. L. Knisley, P. N. Fagin, D. R. Barker, F. Zhang, Microstructure evolution during alpha-beta heat treatment of Ti-6Al-4V. Metall. Mater. Trans. A. 34, 2377–2386 (2003).
[2005Elm] J. W. Elmer, T. A. Palmer, S. S. Babu, E. D. Specht, In situ observations of lattice expansion and transformation rates of α and β phases in Ti–6Al–4V. Mater. Sci. Eng. A. 391, 104–113 (2005).
[2014Sab] M. Saby, E. Massoni, N. Bozzolo, A metallurgical approach to individually assess the rheology of alpha and beta phases of Ti–6Al–4V in the two-phase domain. Mater. Charact. 89, 88–92 (2014).
[2019Sem] S. L. Semiatin, M. Obstalecki, E. J. Payton, A. L. Pilchak, P. A. Shade, N. C. Levkulich, J. M. Shank, D. C. Pagan, F. Zhang, J. S. Tiley, Dissolution of the Alpha Phase in Ti-6Al-4V During Isothermal and Continuous Heat Treatment. Metall. Mater. Trans. A. 50, 2356–2370 (2019).