Pourbaix Diagrams for Complex Alloys
Thermo‑Calc can not only be used to simulate how pure metals interact with pure water or simple aqueous solutions under normal pressure and temperature conditions. It can also be used to calculate how complex alloys and concentrated aqueous solutions interact over a very wide pressure, temperature and composition ranges. This is illustrated with the two examples.
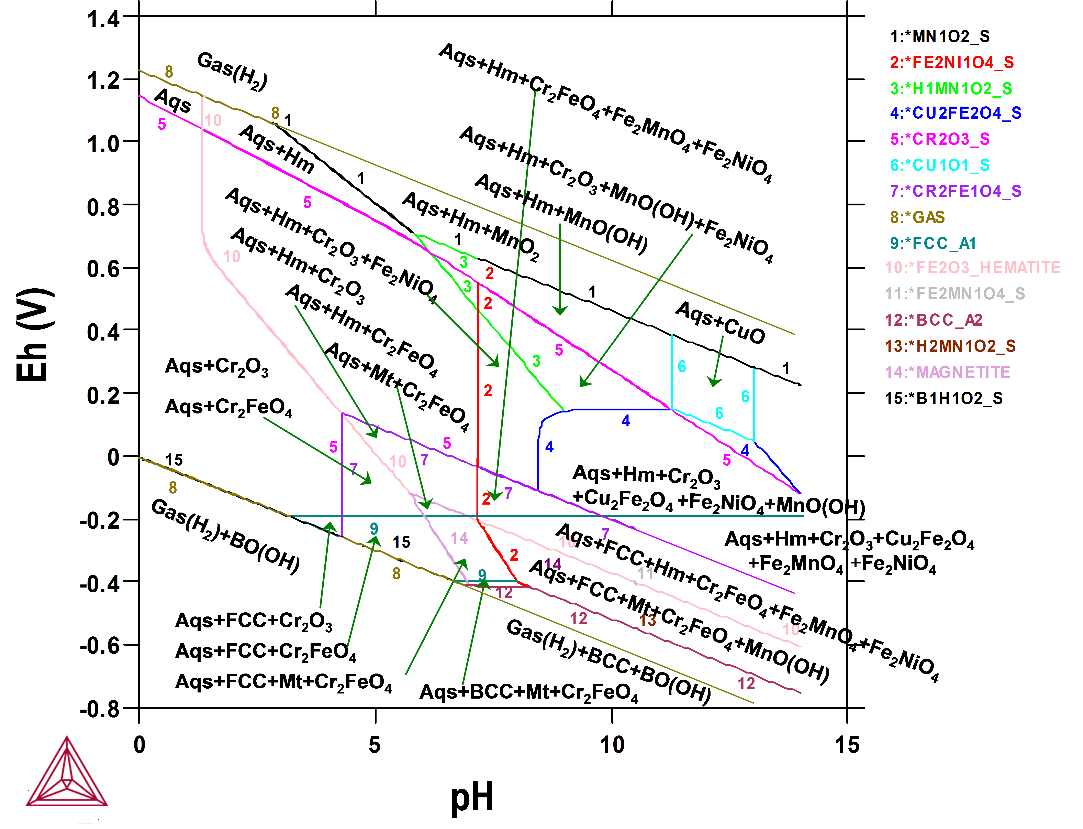
The first example is a Pourbaix diagram calculated for the heterogeneous interaction between 0.001 mole of steel (Fe-7.676Cr-5.0Ni-2.1887Mn-1.0Cu [at%]) and 1 kg of water (and with 1.2 m H3BO3, 0.022 m Li and 0.001 m NH3), at 25° C and 1 bar. This application is particularly useful for safety assessments of nuclear reactors and nuclear waste repositories.
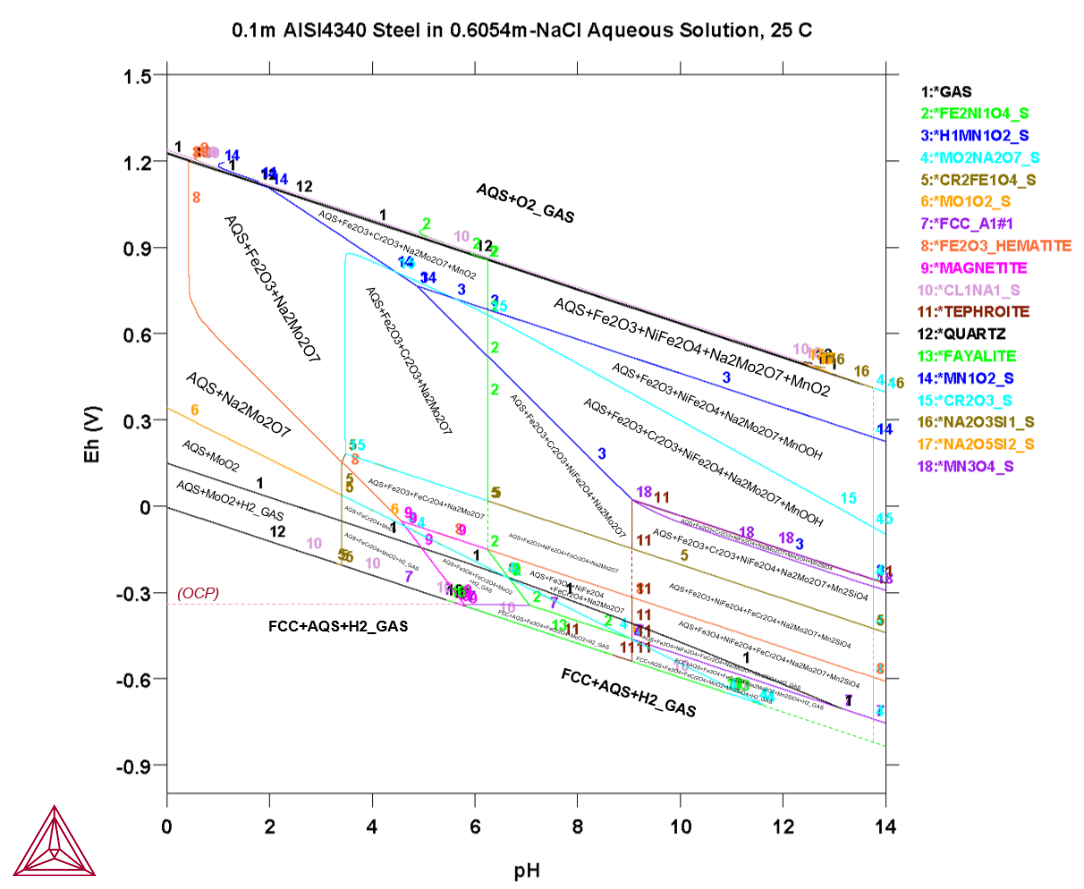
The next example is a Pourbaix diagram calculated for the heterogeneous interaction systems between 0.1 mole of AISI4340 stainless steel (Fe-0.80Cr-1.85Ni-0.70Mn-0.25Mo-0.25Si-0.40C [wt%]) and 1 kg of seawater (with the equivalent of 0.6054 m NaCl), at 25° C and 1 bar. This application is particularly useful for failure analysis of petroleum exploitation industry.