Variations of Pourbaix Diagrams
The shape of a Pourbaix diagram of an alloy or condensed material and the stability relations of various secondary phases depend upon the following system factors:
- Initial amount of the alloy or other condensed materials
- Initial composition of the alloy or other condensed materials
- Initial amount of the interacting aqueous solution phase
- Initial composition of the interacting aqueous solution phase
- Temperature and pressure conditions
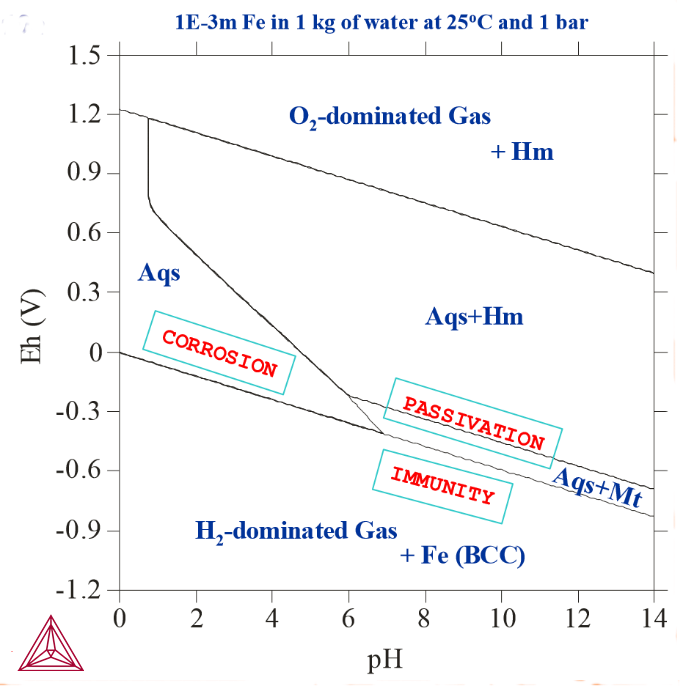
In the interaction system that the diagrams in Pourbaix Diagrams with Gas Phase Excluded and Pourbaix Diagrams with Gas Phase Included are based on, the initial amount of pure Fe that was taken to have effectively reacted with 1 kg of pure water at 25° C and 1 bar was 0.001 m. The following diagrams are Pourbaix diagrams of Fe where other initial amounts of pure Fe have been used, or where the interacting aqueous solution compositions have been alternated, or the temperature and/or pressure have been changed. Gaseous mixture phases have been included in all the calculations that these diagrams are based on.
Figure 1: 1E-3 m Fe actively reacted with 1 kg of pure water at 25° C and 1 bar (as in the calculation in Pourbaix Diagrams with Gas Phase Included).
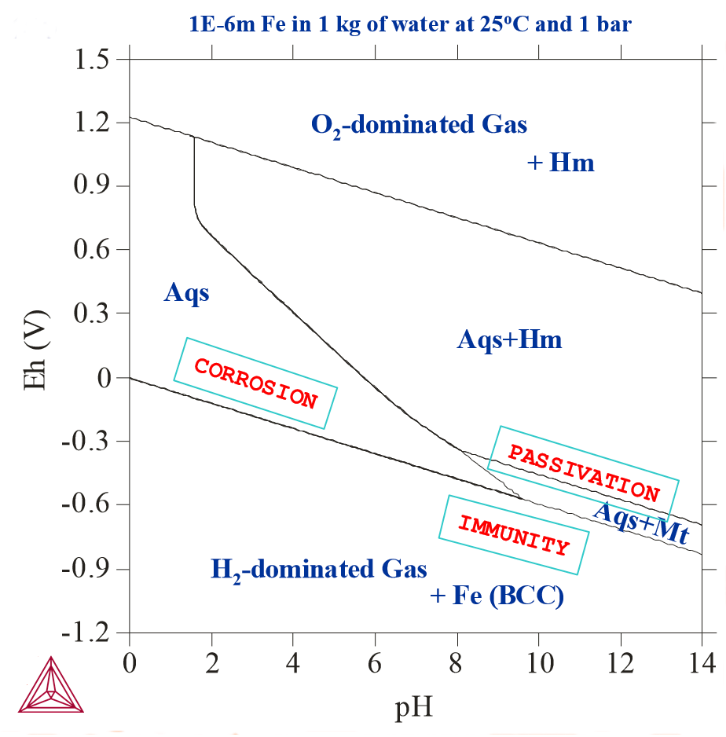
Figure 2: 1E-6 m Fe actively reacted with 1 kg of pure water at 25° C and 1 bar. Note that the active metal corrosion region gets enlarged as the initial Fe amount decreases from 1E-3m to 1E-6m.
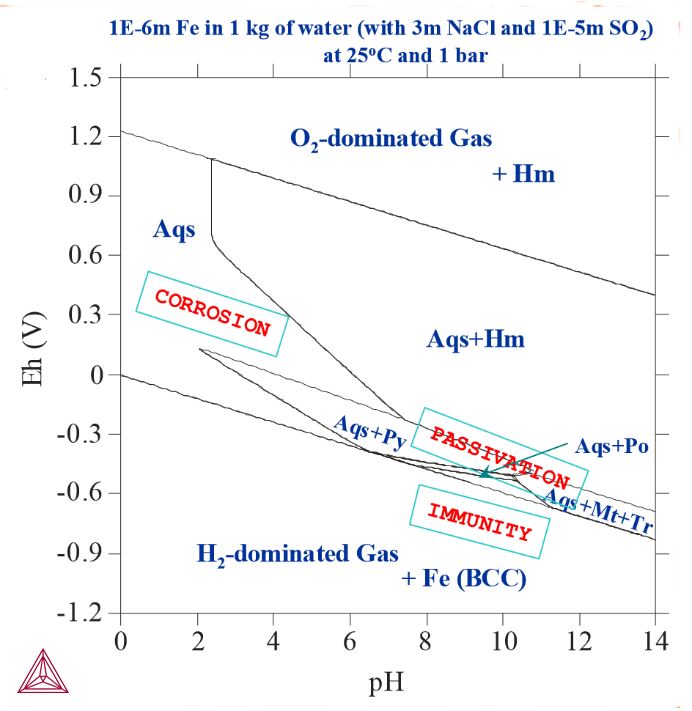
Figure 3: 1E-6 m Fe actively reacted with 1 kg of water, 3m NaCl and 1E-5 m SO2 at 25° C and 1 bar. Introducing SO2 into the system leads to the formation of various metal-sulphides (Py-pyrite, Popyrrhotite, Tr-troilite). In addition, the passivation region becomes larger.
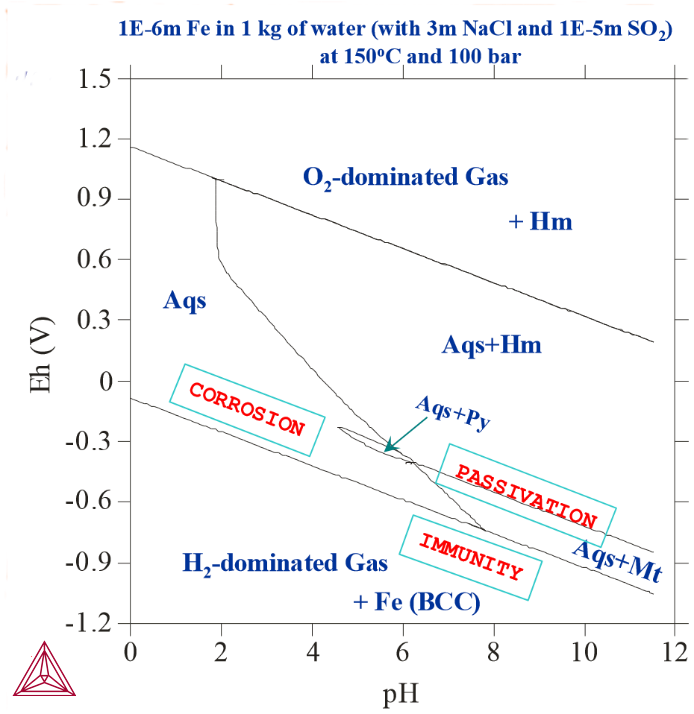
Figure 4: 1E-6 m Fe actively reacted with 1 kg of water, 3m NaCl and 1E-5 m SO2 at 150° C and 100 bar. Here, changing the temperature and pressure affects the stability fields of various Fe-oxides/sulphides.
As you can see from the figures, the aqueous-gas phase boundaries shift as the initial bulk compositions, pressure and temperature conditions in the interaction system change.