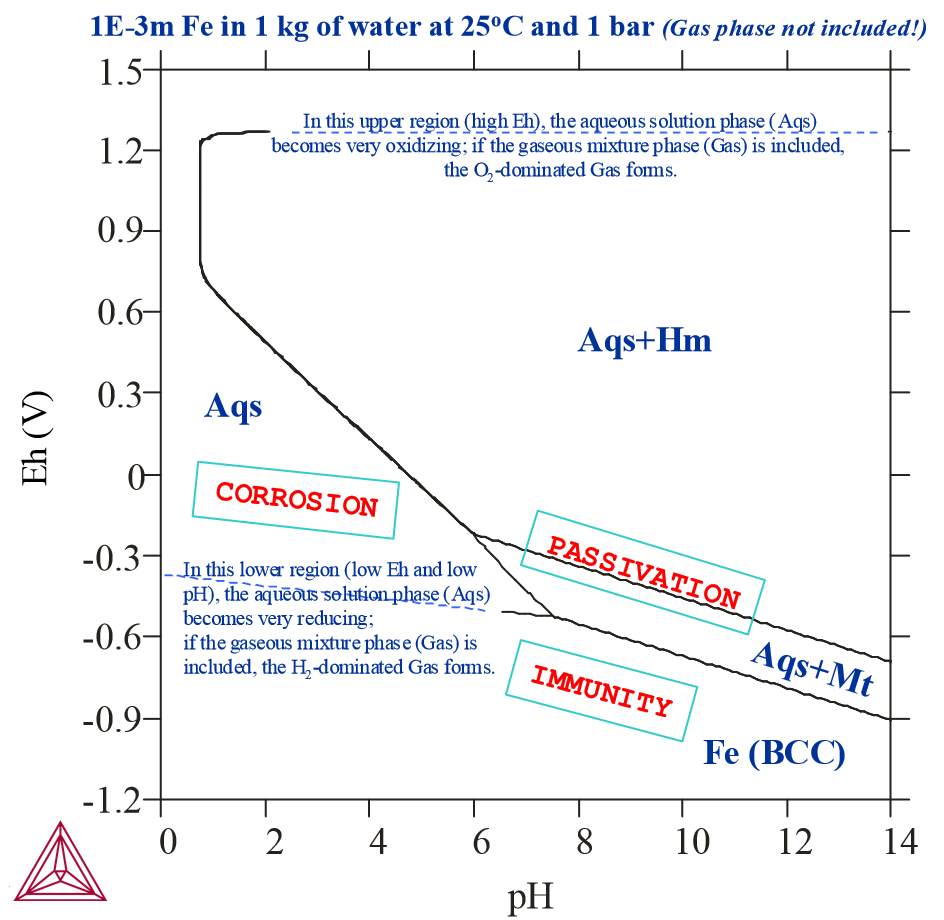
Pourbaix Diagrams with Gas Phase Excluded
This is an example of a Pourbaix diagram of Fe, in which the gas phase has not been considered in the calculation. The system has 0.001 mole of Fe in 1 kg of pure water at a temperature of 25oC and a pressure of 1 bar. Magnetite (Mt, Fe3O4) and hematite (Hm, Fe2O3) co-exist with the Fe-containing dilute aqueous solution. The formation of magnetite and hematite represent the passivation of iron in the upper right pH-Eh field.
If pH is low and Eh is relatively high, then Fe eventually completely dissolves into the aqueous solution phase. Under conditions of low Eh, Fe remains in its stable solid state, the BCC phase, and neither dissolves into water nor into alternates to Fe (the immunity of Fe).