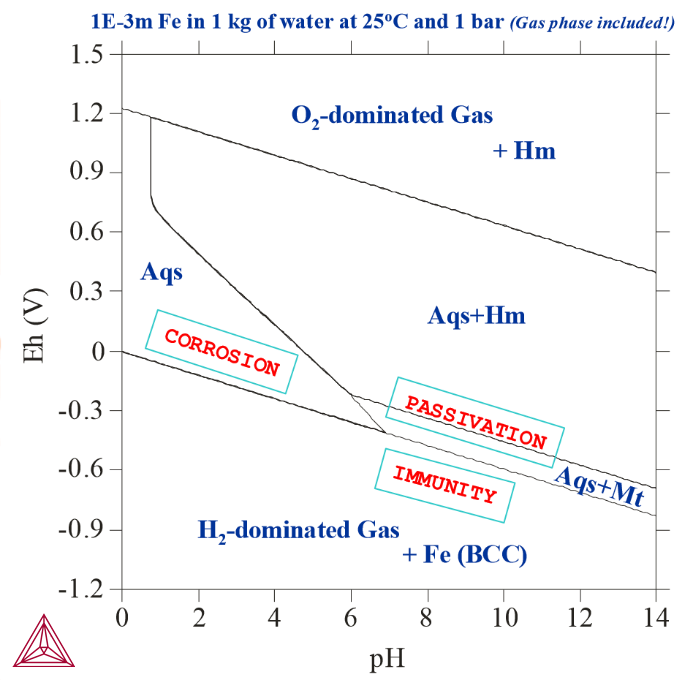
Pourbaix Diagrams with Gas Phase Included
With all the possible redox reactions involving the aqueous solution phase and gaseous mixture phase being considered in the equilibrium system, that is, the gaseous mixture phase is included in the calculation, the Pourbaix diagram of Fe in a system with 0.001 mole of Fe in 1 kg of pure water at 25oC and 1 bar.
Comparing this Pourbaix diagram with that from Basic Settings and Definitions, you can see that both diagrams are for the same interaction system under the same temperature-pressure condition, but the present diagram shows the results of calculations where the gaseous mixture phase has been included. It presents the complete Pourbaix diagram for the defined interaction system, in a full thermodynamic equilibrium. In the upper region (high Eh) and lower region (low Eh), the aqueous solution phase is oxidised and reduced to the O2- or H2-dominated gas phase. Hematite coexists with the O2-dominated gas, while Fe-BCC is stable with the H2-dominated gas. The stability field of magnetite may extend slightly into the H2-dominated gas region, where it coexists with the gaseous mixture instead of the aqueous solution. Its boundary with Fe (BCC) cannot be drawn due to the absence of aqueous solution phase.
It is not possible to calculate the pH value in both the O2- and H2-dominated gaseous mixture phase stability regions where the aqueous solution phase is absent. Hence, in a normal Pourbaix diagram, no line is drawn that indicates a phase boundary between the gas phase and the metallic phases or secondary phases (that is, metal-oxides).