About the ENTER_PARAMETER Command
For the PARROT Module, this command is the same and is described here. See ENTER_PARAMETER for the command details.
In the descriptions of the standard thermochemical properties and special physical properties for a phase, there are a number of parameters which may depend on the temperature and pressure. The expressions for these parameters can be given in a free form as a sum of terms with powers of T and P and may also include the natural logarithm and exponential function. This type of expression is called TP-functions. Identical parameters (in terms of parameter-names) are stored only once in the GIBBS workspaces.
The composition-dependence of the Gibbs energy is described in the GIBBS module by the internal data structure, which is created when the phase is entered. The Gibbs energy of a phase is always referred to one formula unit of the phase, i.e. the amount derived from the number of sites (i.e. the stoichiometric coefficient) for each sublattice. If vacancy is a constituent of a sublattice, the amount of matter per formula unit of the phase may vary with composition.
Gibbs Energy System (GES) Commands
A valid parameter should have the general form of:
<identifier>(<phase>, <constituent array>; <digit>) <xxx> <expression> <yyy> <keyword Y or N> <zzz> !
The identifier must be followed by an opening parenthesis, a phase name, a comma and a constituent array. Optionally, the constituent array can be followed by a semicolon and a digit. The parameter name is terminated by a closing parenthesis. The parameter form is defined as:
<identifier>is the parameter type;<phase>is the phase name (maximum 24 characters);<constituent array>is the specific constituent array in the phase;<digit>is the degree of composition-dependent interaction contribution (an integer number from 0 through 9), that is only for excess energy (L), Curie temperature (TC) and Bohr magneton number (BMAGN), as well as for volume-related parameters (V0 or VA or VC or VK); if it is valued as zero, or if it is for the standard Gibbs energy (G) for which the degree is always zero, it can be omitted;<expression>is the mathematical relation to describe the parameter;<xxx>and<yyy>are the low and high temperature limits respectively for the applicable temperature range of the parameter expression;<keyword Y or N>is the indicator on if there is continuation for the parameter expression or not;<zzz>is the reference index/number for the assessment of this parameter;- The exclamation point
!is used to indicate that the current parameter definition is ended.
The GES parameter name has a general form of:
<identifier>(<phase>,<constituent array>;<digit>)
Examples of parameter names:
G(GAS,C1O2): The Gibbs energy of formation of a CO2 molecule in gas.G(FCC,FE:VA): The Gibbs energy of formation of fcc Fe with interstitials.L(LIQ,Fe,Cr;0: The regular solution parameter for Fe and Cr in liquid.L(LIQ,Fe,Cr;1): The sub-regular solution parameter.TC(BCC,Fe:Va): The Curie temperature of bcc Fe.BMAGN(BCC,Fe:Va): The Bohr magneton number parameter of bcc Fe.
The parameter name consists of several parts. The first is a type-identifier and these can be used:
G: Standard energy parameter (Gibbs energy of formation) or for interaction parameters;L: Excess energy parameter (Gibbs energy of interaction) always used for interaction parameters;TC: Curie temperature for magnetic ordering;BMAGNorBM: Bohr magneton number for magnetic ordering (or Born function ωPr,Tr for aqueous solute species).VO: Molar volume at 298.15 K and 1 bar (a numeric value only);VA: Integrated thermal expansivity;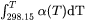
VC: High-pressure fitting parameter;VK: Isothermal compressibility.
For more information about the high pressure volume, see X.-G. Lu, M. Selleby, B. Sundman, "Implementation of a new model for pressure dependence of condensed phases in Thermo‑Calc", Calphad. 29, 49–55 (2005).
When necessary quantities as H (enthalpy), S (entropy), V (Volume), F (Helmholtz energy), etc., can be calculated from the Gibbs energy.
Specifying the PHASE NAME in uppercase is recommended; however, if you prefer to write it as a mixture of uppercase and lowercase, it automatically converts all lowercase to uppercase, as the GIBBS module only recognizes uppercase phase names. It is important that if a phase bears a legal phase-type (among G, A, Y, L, I, F and B) in its phase definition (already by the PHASE keyword; such as GAS:G, LIQUID:L,SLAG:L, IONIC-LIQ:Y, SPINEL:I, FCC_L12:F, HCP_D021:F, BCC_B2:B, AQUEOUS:A), such a valid phase-type code should not be attached to the phase name when ENTER_PARAMETER is executed.
The constituent array consists of a list of constituent names. Interaction parameters have two or more constituents from the same sublattice separated by a comma. If the phase has sublattices, at least one constituent in each sublattice must be specified. The constituents in different sublattices must be given in sublattice order and are separated by a colon.
After the component array, a sub-index digit can be specified after a semicolon. This digit must be in the range 0 to 9. The interpretation of the sub-index depends on the excess energy model used for the phase. If no semicolon and digit are given, the sub-index value is assumed to be as zero.
The excess energy parameters, e.g. the regular/subregular (binary) parameter or ternary parameters, are multiplied with two or more fractions of the constituents from the same sublattice of the solution phase. These additional constituents must be given as interacting constituents (as the following prompt).
Solution phases with sublattices can have interacting constituents in each sublattice.
An interaction parameter, which is used to describe the excess term of a quantity, must have two or more constituents that interact with each other on a specified sublattice site of the given phase. It is arbitrary which of these constituents is given as the first constituent and what is given as the interacting constituents. The software always sorts the constituents (in each sublattice) in alphabetical order when the parameter name is written as a prompt (for entering its parameter value) and when the parameter is listed (using the GIBBS commands LIST_PARAMETER or LIST_PHASE_DATA). This is important for all asymmetric interaction parameters where the sign of the interaction parameter must depend on the appearance order.
Use an asterisk* to denote that the excess interaction parameter is independent of the constituents of a specific sublattice. For example, L(FCC_L12,AL,NI:*) means that the interaction parameter is for the binary interaction between constituents AL and NI on the first sublattice in the FCC_L12 solution phase, while it is independent of all constituents on the second sublattice. An interaction parameter in the list of constituents is always added to the Gibbs energy and the asterisk * is calculated with the term of [1∑y(specified constituents)], which implies that in an A-B binary system these L parameters are identical (but in higher-order systems, these are different):
L(phase,A,B)is multiplied with X(A)*X(B)L(phase,A,*)is multiplied with X(A)*(1-X(A))L(phase,B,*)is multiplied with X(B)*(1-X(B))
If you press <Enter> when you are asked for a parameter name or if you have improperly input the entire parameter name, you are asked for each of these items in the name.