Metastable Phases / Precipitates
Most important precipitates that form during aging treatments and casting of aluminum alloys are metastable, except for a few such as Al3Sc and Al3Er (both modeled as AL3X). This example uses the TCS Al-based Alloy Database (TCAL).
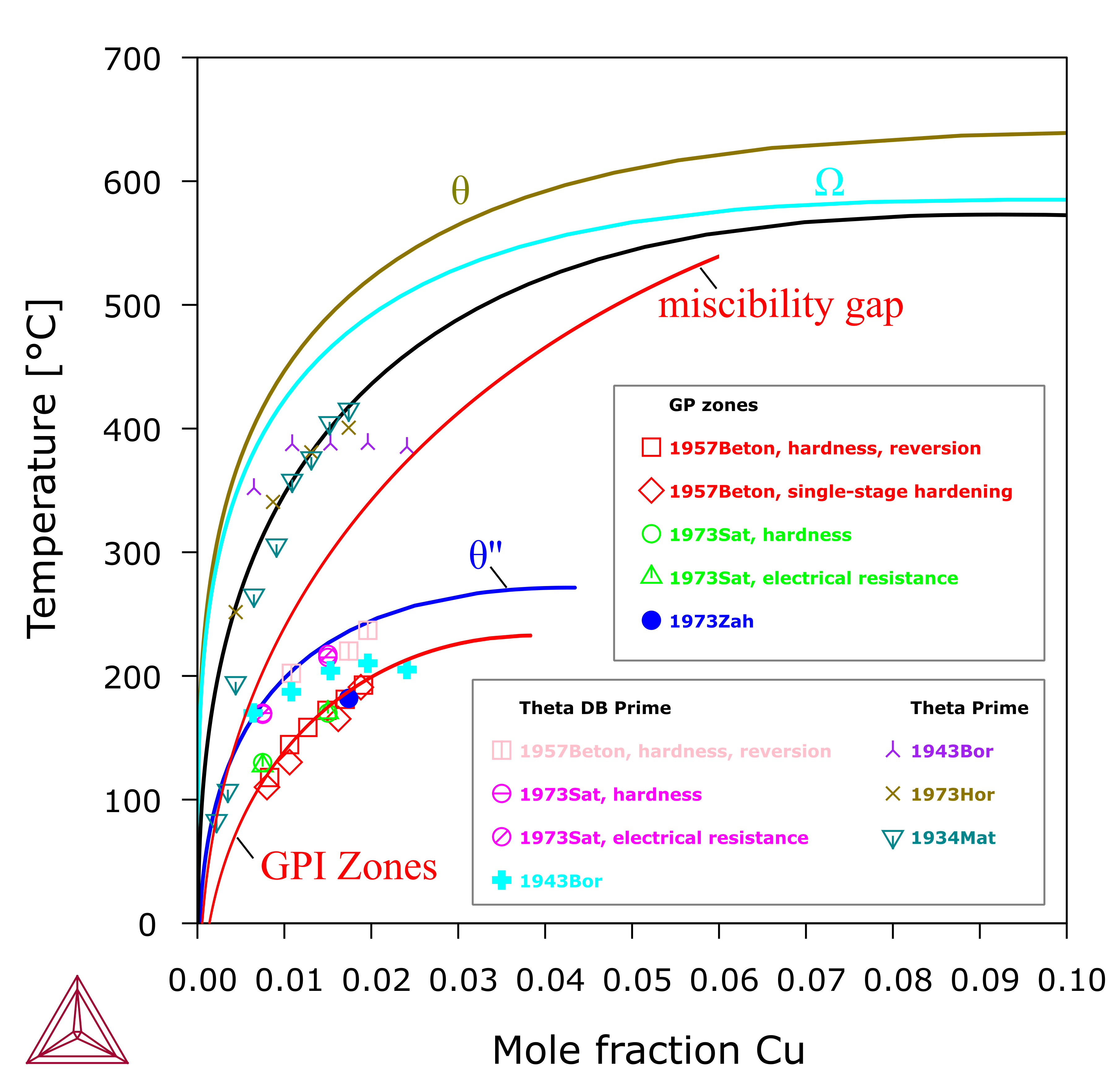
Figure 1: Calculated Al-Cu (Al) solvus curves equilibrated with θ, Ω, θ', θ'' (or GPII zones) and GPI zones, respectively. A metastable miscibility gap of fcc_A1 is shown. The GPI zones are modeled as the second composition set of fcc_A1, i.e. fcc_A1#2. It is assumed that experimentally observed GPI zones are usually tiny (say < 3 nm), so the interfacial and elastic energy are considered. The line for GPI zones is calculated with adding +800 J/mole-atoms to the energy of fcc_A1#2 [2014Che].
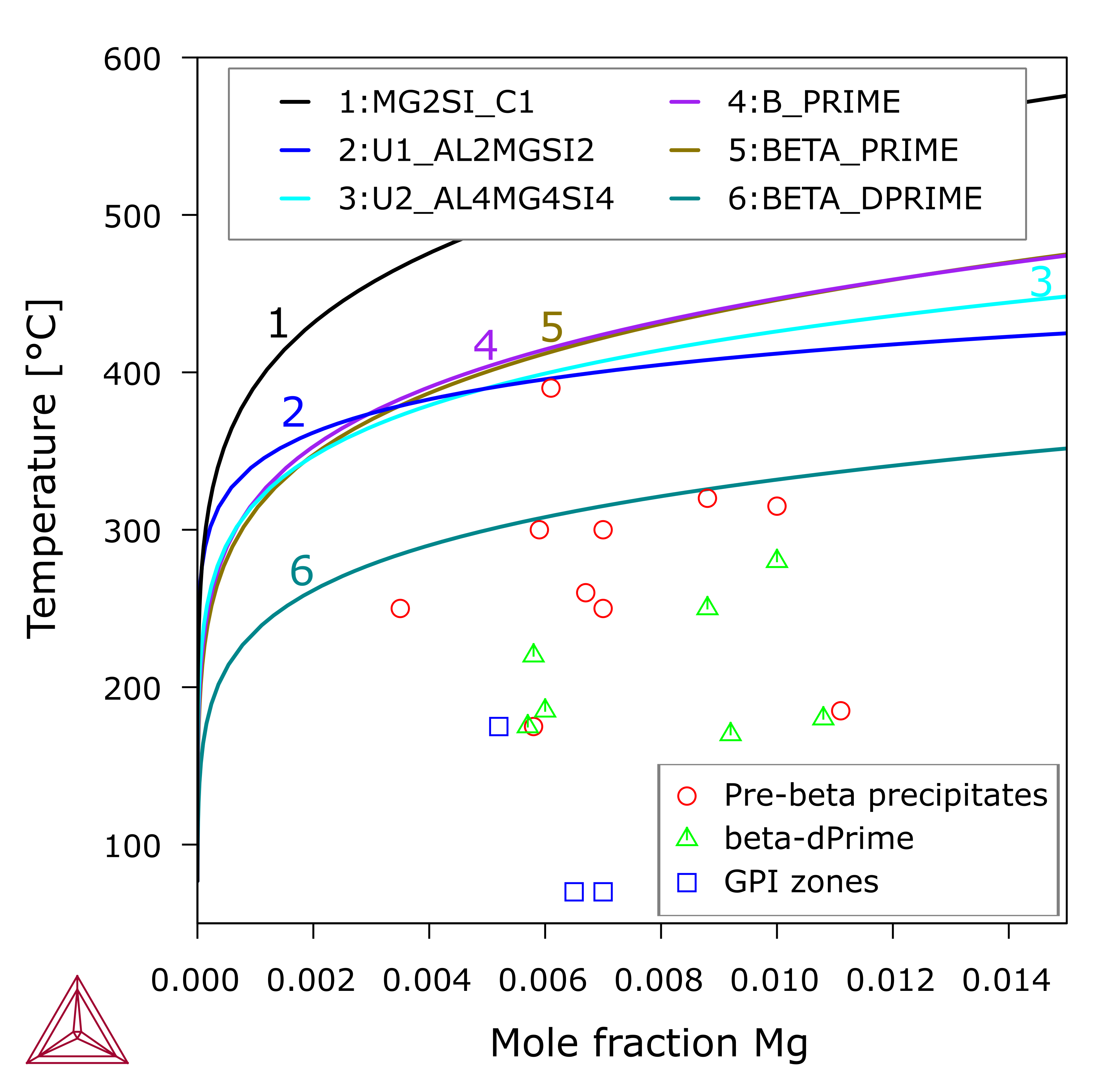
Figure 2: Calculated fcc_A1 solvi in alloys at 0.73 at.% Si and varying Mg content, relative to different Al-Mg-Si precipitates, including the stable β-Mg2Si phase, pre-β precipitates (β', U1, U2 and B')and the pre-β' precipitate, i.e. β''. The symbols indicate certain precipitates have been experimentally observed in alloys of given compositions and aged at given temperatures [2014Che].
Reference
[2014Che] H.-L. Chen, Thermodynamic modeling of metastable precipitate phases in Al-Cu, Al-Fe, Al-Mg-Si, and Al-Mg-Zn based alloys (Stockholm, Sweden, 2014), unpublished work.