Ternary System Examples
These ternary system examples using the TCS High Entropy Alloys Database (TCHEA) and for Co-Cr-Ni, Cr-Fe-Ni, and Nb-Ti-V show the phase stabilities in two dimensional plots, i.e. isothermal or vertical sections. It can be used to directly study medium entropy alloys (MEAs) or guide the design of high entropy alloys (HEAs).
The CALPHAD Method and the Thermo‑Calc Databases. Also visit the video tutorials on our website or our YouTube playlist.
Isothermal sections and liquidus projections for assessed ternary systems can be calculated using the Ternary Calculator in Graphical Mode or the TERNARY_DIAGRAM module in Console Mode. In Thermo‑Calc press F1 to search the Help for more information.
Co-Cr-Ni
A vertical section, which is sometimes referred to as an isopleth, is a diagram showing the phase equilibria along a specific composition line and over a temperature range. These are often used to interpret the impact of the variation of composition on phase equilibria over temperatures or comparing the phase formation in a series of compositions during heating or cooling. Phase regions and boundaries among these are shown in a two-dimensional (2D) diagram.
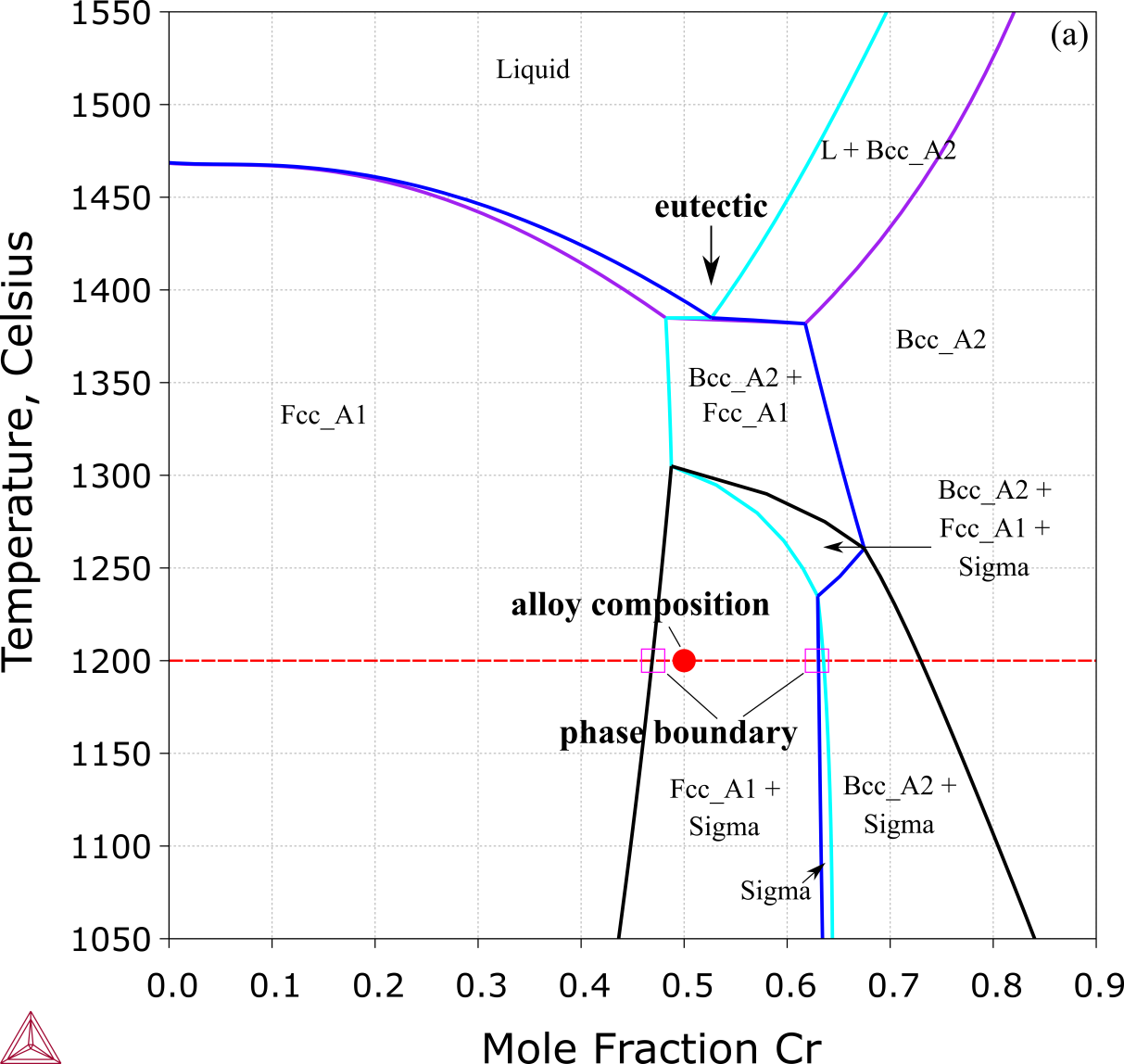
Such a 2D diagram is similar to a binary phase diagram, but with an important difference: the level rule does not hold there. In other words, the phase boundaries of a two-phase region at specific temperatures in an ordinary isopleth do not correspond to the equilibrium compositions of the two phases. As shown in Figure 1, the alloy Co0.25Ni0.25Cr0.50 is located in FCC_A1 + Sigma two-phase region at 1200 °C, which is adjacent to the FCC_A1 and Sigma single-phase regions. The boundaries just mark the end of the two-phase region, and the compositions are different from the equilibrium compositions of the two phases.
Figure 1: Equilibrium calculation of the extensively studied medium entropy alloy (MEA), the Co-Cr-Ni ternary system. This plot shows a vertical section from Cr0.5Ni0.5 to Cr [2018Che].
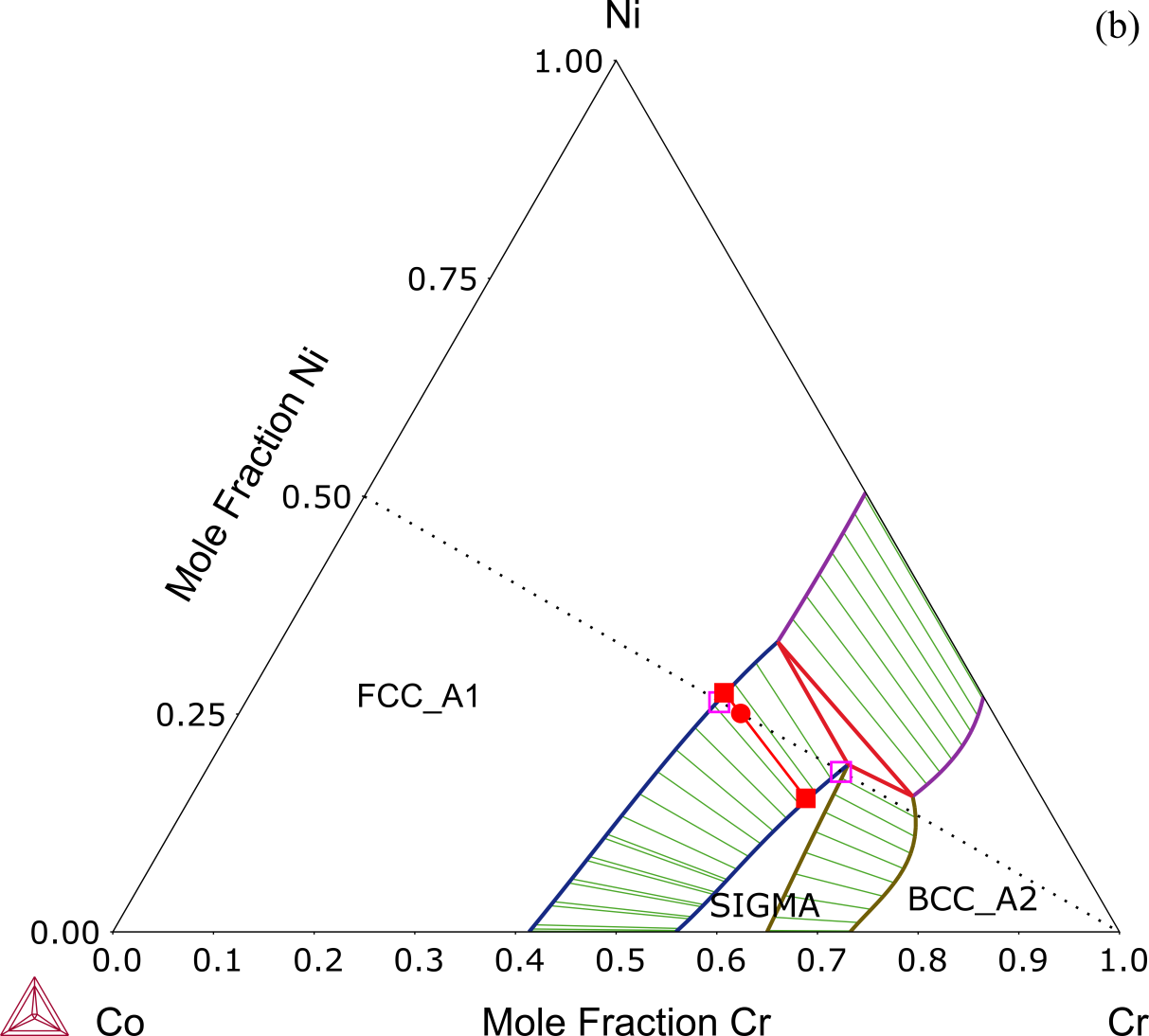
The tie-line, which stands for the composition line connecting the two equilibrium phases, is not confined within the vertical section. This can be clearly shown with an isothermal section at the same temperature as in Figure 2.
Figure 2: Equilibrium calculation of the extensively studied medium entropy alloy (MEA), the Co-Cr-Ni ternary system. This plot shows an isothermal section at 1200 °C. The alloy composition locates at Co0.25Ni0.25Cr0.50 [2018Che].
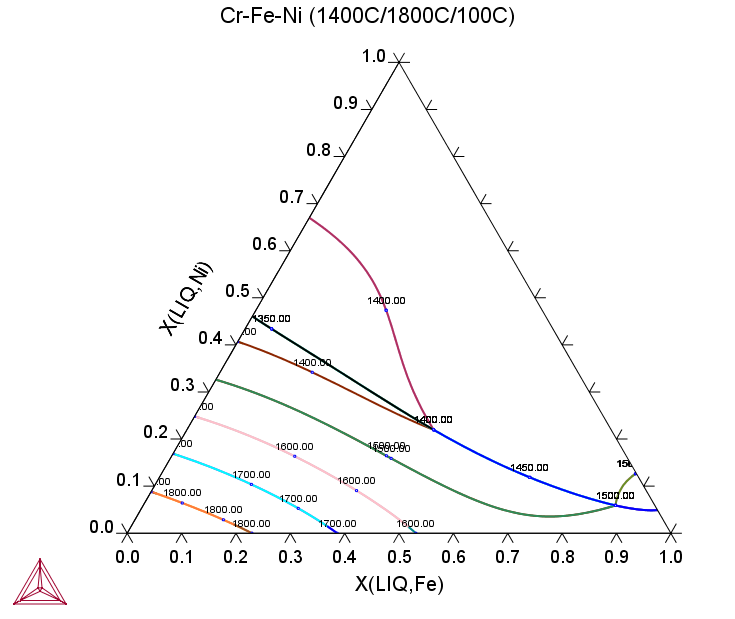
Cr-Fe-Ni
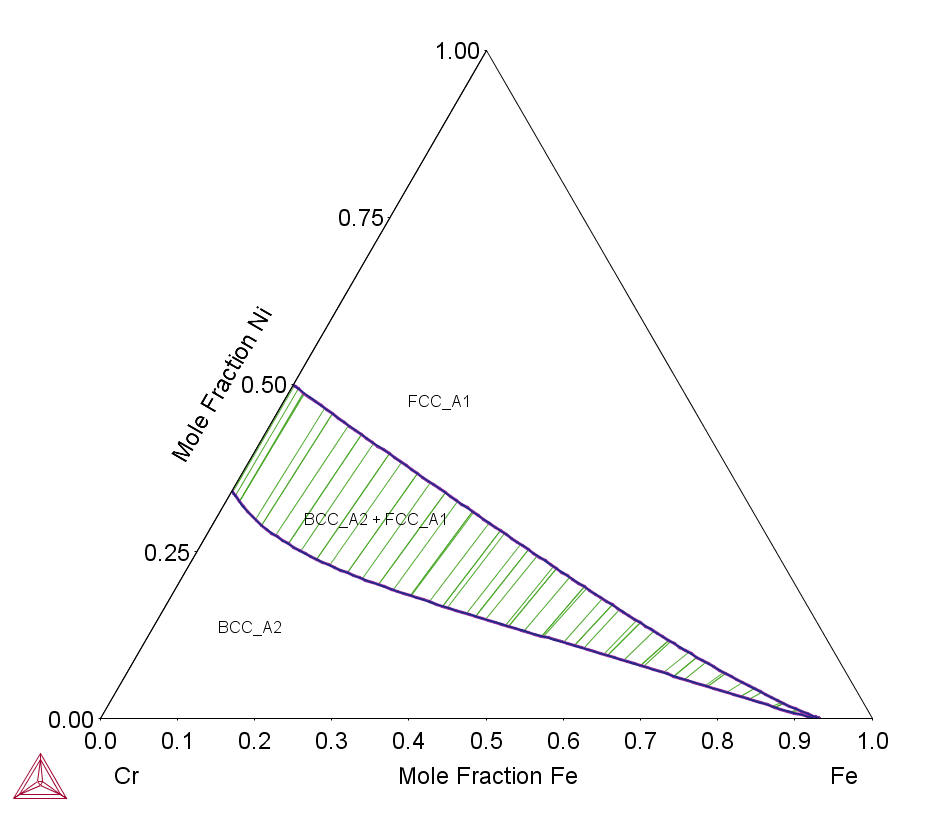
The Cr-Fe-Ni ternary provides a model system where the primary phase could either be BCC or FCC. The following equilibrium calculations—an isothermal section, the liquidus monovariant curve and the liquidus projection overlapping with a series of isothermal sections—can be used to design MEAs or HEAs with single phase (BCC or FCC) or dual-phase (BCC + FCC).
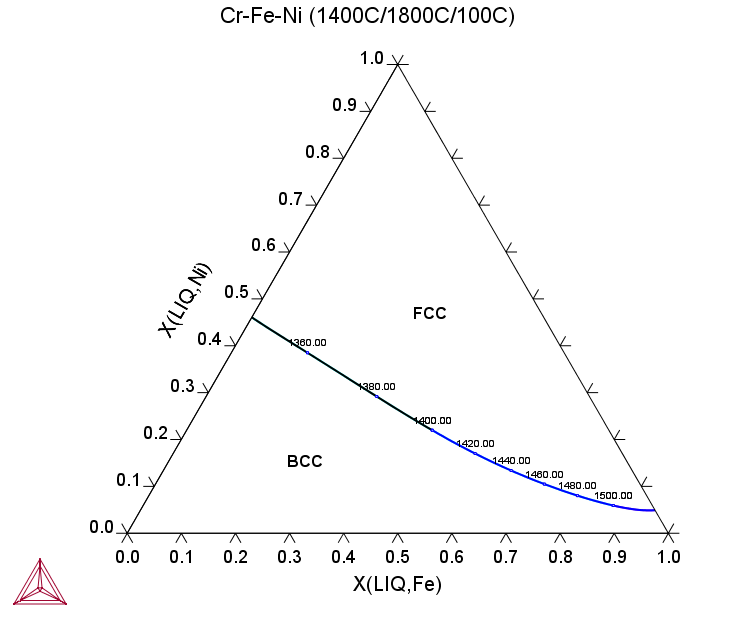
Figure 4: Equilibrium calculation of the Cr-Fe-Ni ternary showing a liquidus surface projection. The temperature (°C) value is plotted along the monovariant reaction curve. The primary phase shifts from BCC in Cr-corner to FCC in Ni-corner.
Figure 5: Equilibrium calculation of the Cr-Fe-Ni ternary with a liquidus projection overlapping with series of isothermal sections of the Cr-Fe-Ni ternary.
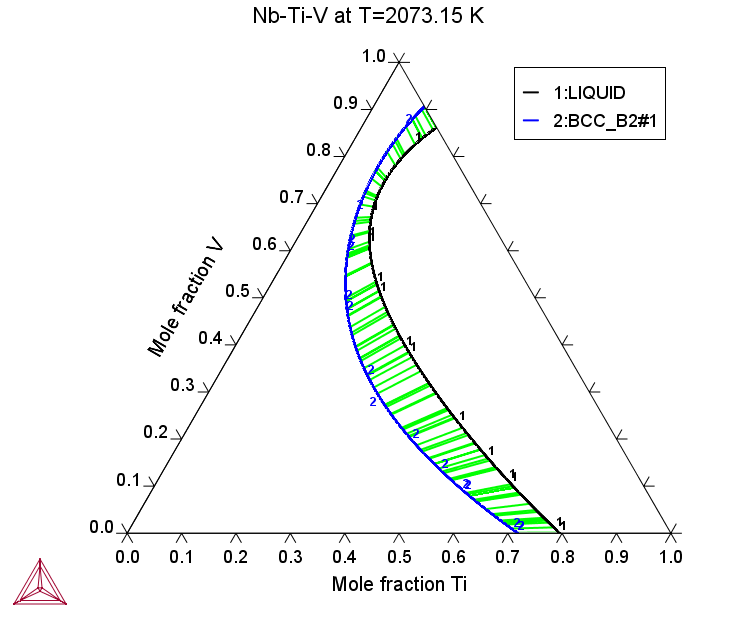
Nb-Ti-V
The Nb-Ti-V ternary system is important for the refractory BCC HEAs.
Reference
[2018Che] H.-L. Chen, H. Mao, Q. Chen, Database development and Calphad calculations for high entropy alloys: Challenges, strategies, and tips. Mater. Chem. Phys. 210, 279–290 (2018).