Hydrogen Storage Alloys
Magnesium and magnesium alloys have been intensively studied for decades as hydrogen storage materials. Magnesium could form MgH2 with hydrogen. MgH2 has a very high hydrogen storage capacity, but has obvious disadvantages in both thermodynamics and kinetics. Researchers have attempted to optimize the thermodynamic and kinetic properties of MgH2 via various techniques, e.g. via alloying, using catalytic additives, deforming, and nanotechnology, and so forth.
As for the alloying route, investigated alloys include Mg-Ni, Cu-Mg, Mg-Nd, Ce-Mg, and La-Mg, and so forth.
In TCS Mg-based Alloys Database (TCMG) starting with version 6 (TCMG6), eight binaries (Ce-H, Cu-H, La-Zn, H-La, H-Mg, H‑Nd, H-Ni, and H-Zn) and five ternaries (Ce-H-Mg, Cu-H-Mg, H-La-Mg, H-Mg-Nd, and Mg-H-Ni) are modeled for application to hydrogen storage. This is in to the relevant systems already modeled in versions TCMG5 and earlier.
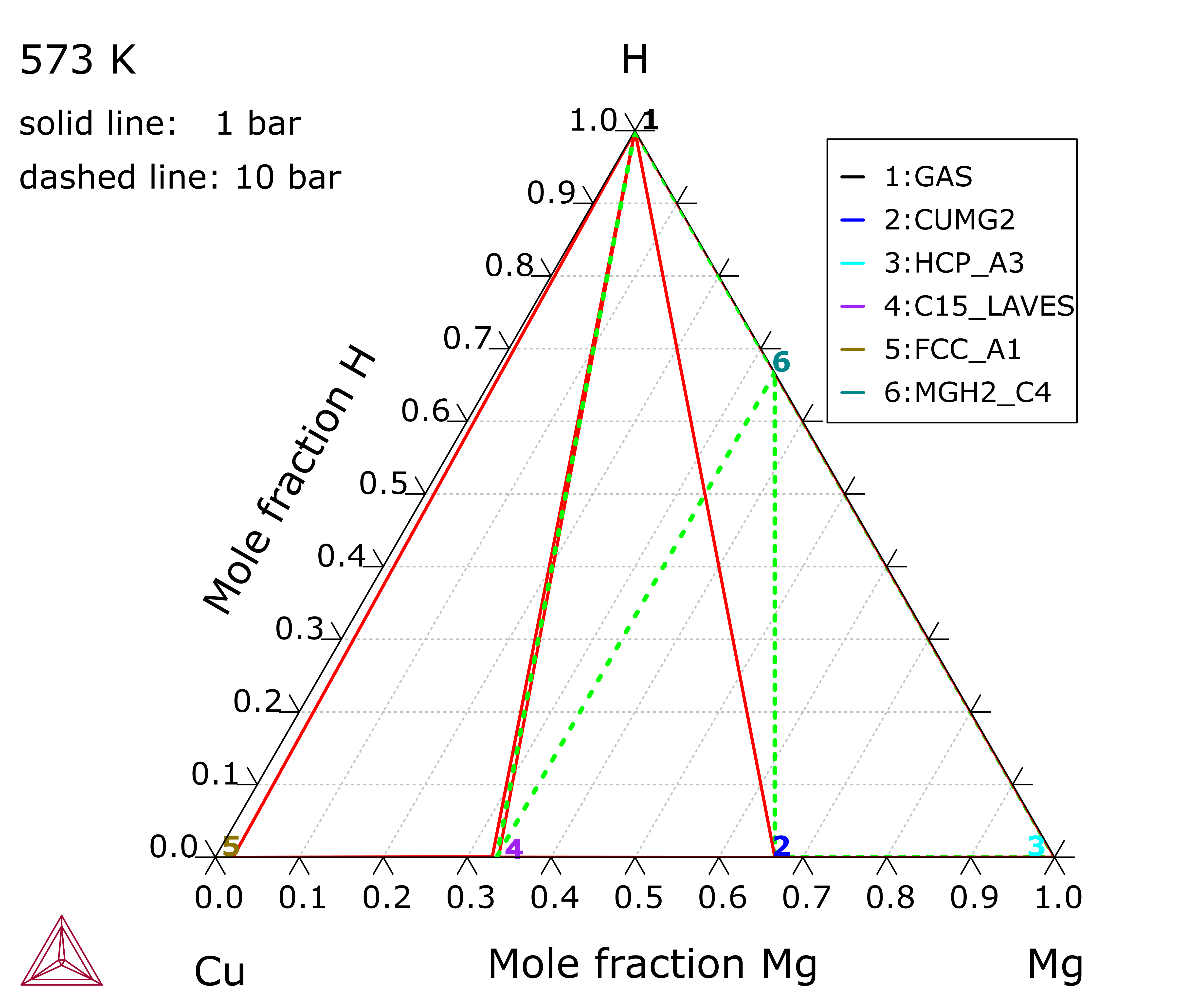
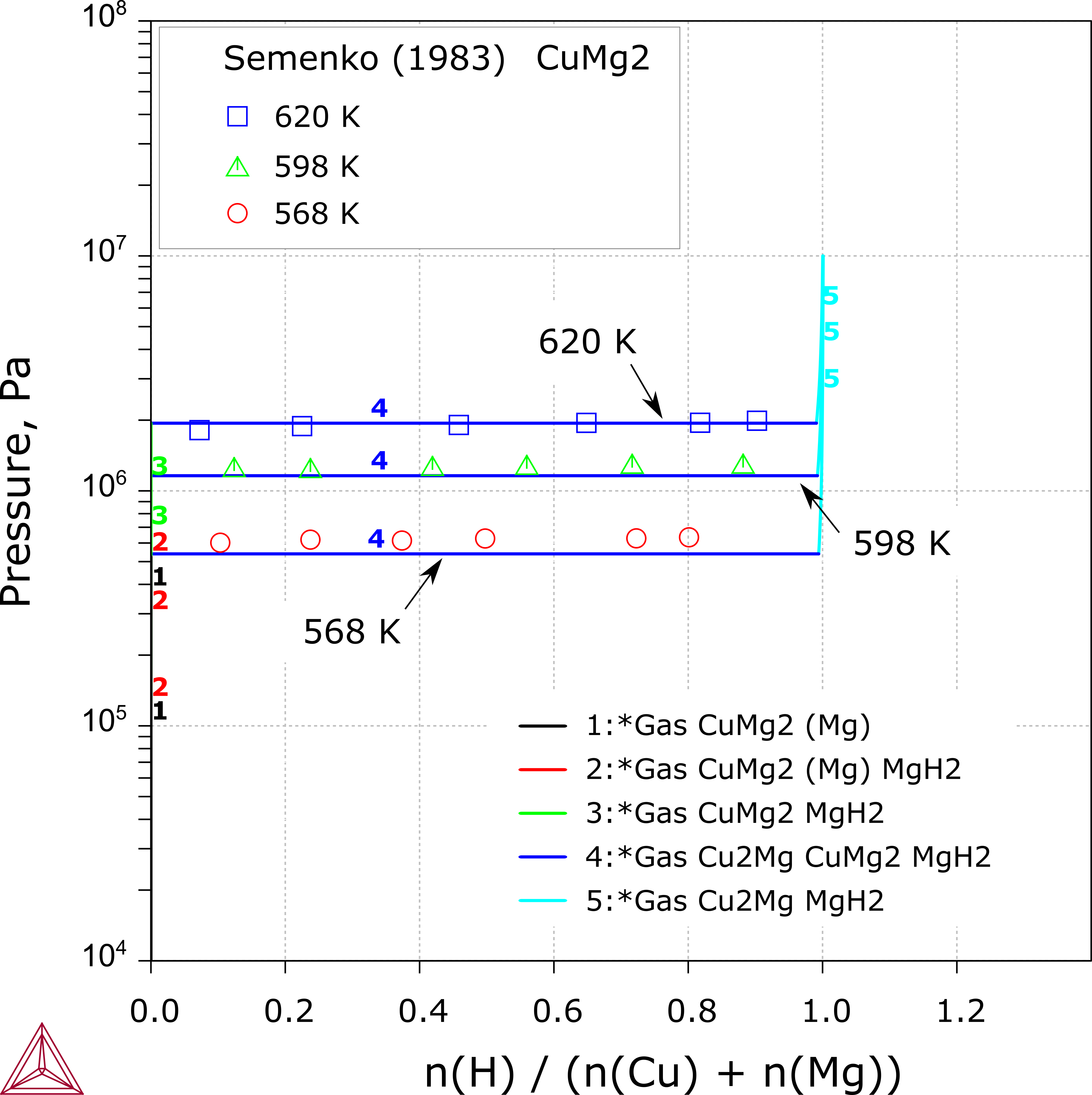
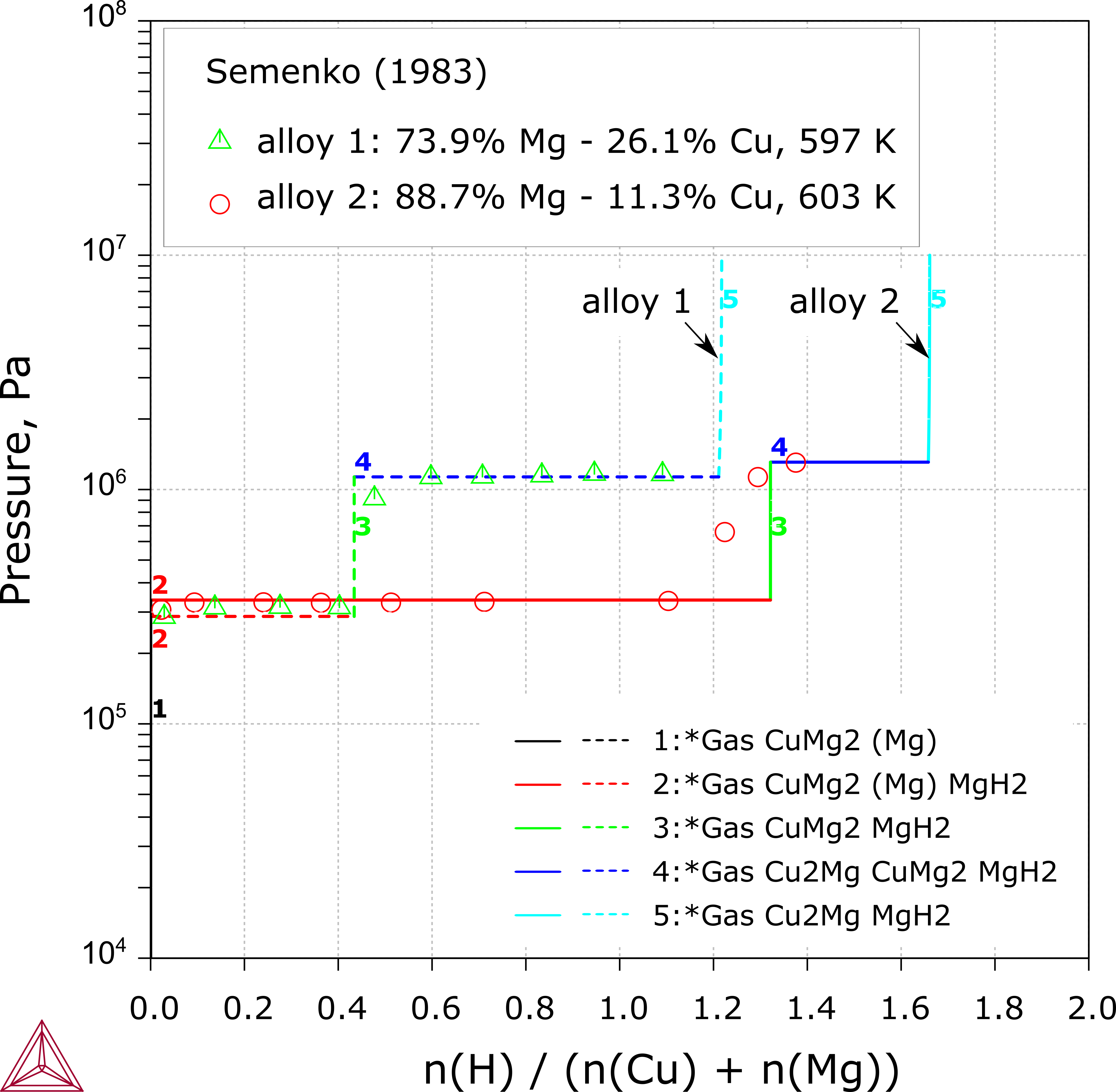
Cu-Mg-H
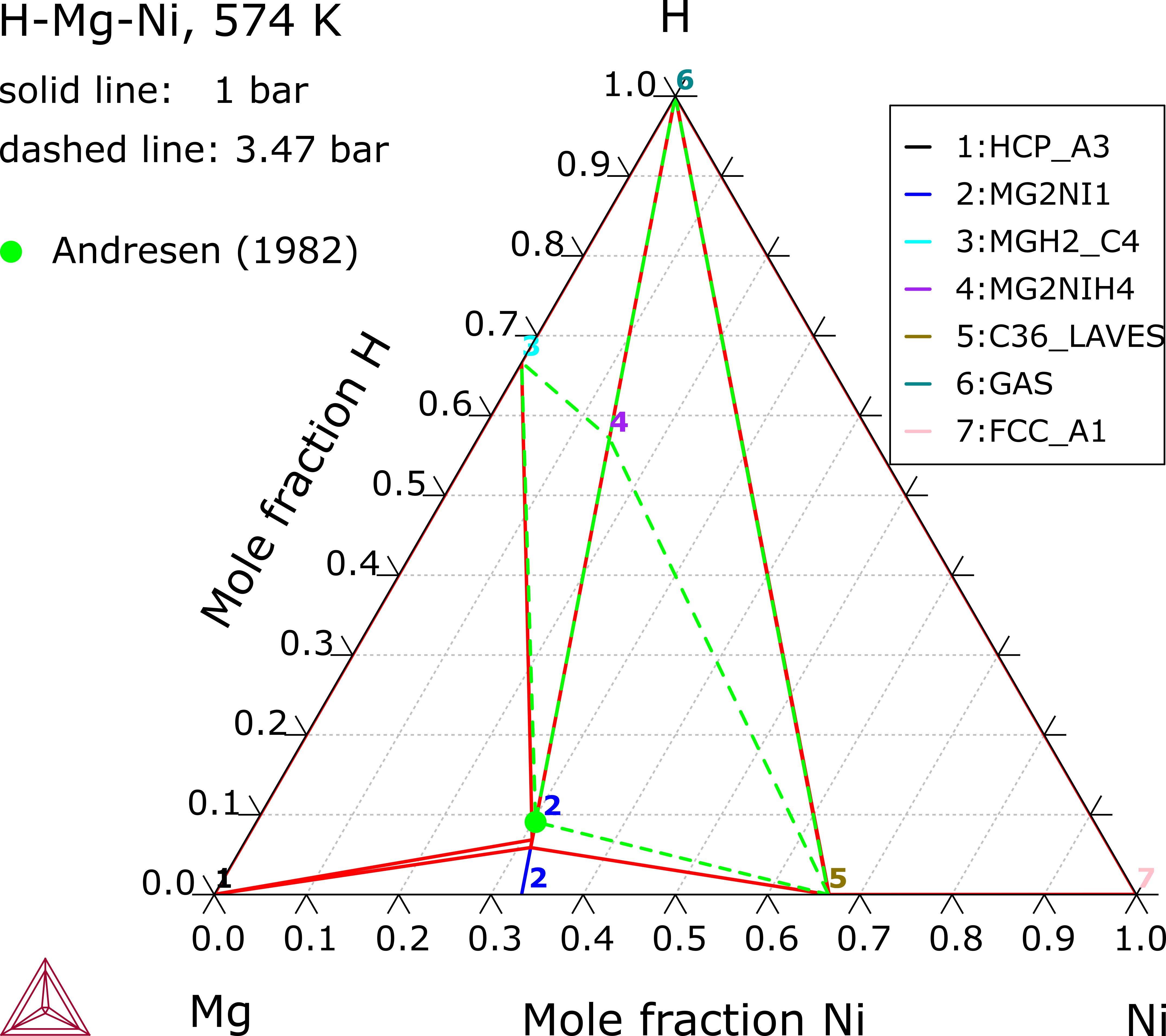
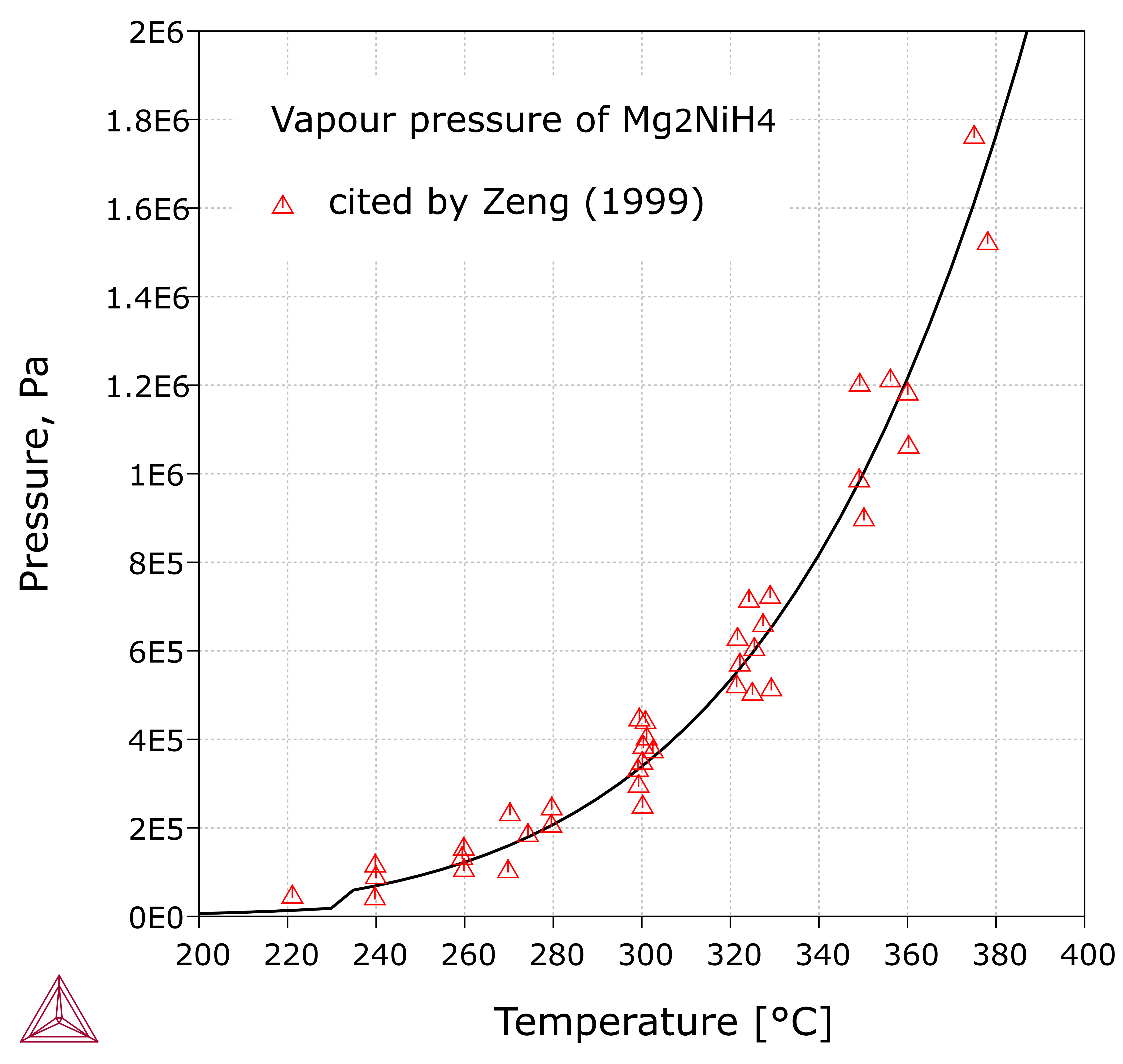
Mg-H-Ni
References
[2020aChe] H.L. Chen, Modeling of Cu-H-Mg in TCMG6, Thermo‑Calc Software (2020).
[2020bChe] H.L. Chen, Refinement of Mg-H-Ni in TCMG6, Thermo‑Calc Software (2020).