Ternary Phase Diagrams
You can use the TCS Mo-based Alloys Database (TCMO) to plot ternary phase diagrams in Thermo-Calc. Each ternary system is critically assessed to reproduce the phase diagram and thermodynamic properties data available in the literature. The following examples show a selection of the key systems that are the building blocks of the database itself when applying the CALPHAD method.
The CALPHAD Method and the Thermo‑Calc Databases. Also visit the video tutorials on our website or our YouTube playlist.
When working in Thermo‑Calc with ternary diagrams you use either the Ternary Calculator (in Graphical Mode) or the Ternary module (in Console Mode). The fundamental calculation engine is the same but you access the settings in different ways.
B-Mo-Si: Isothermal Section and Liquidus Projection
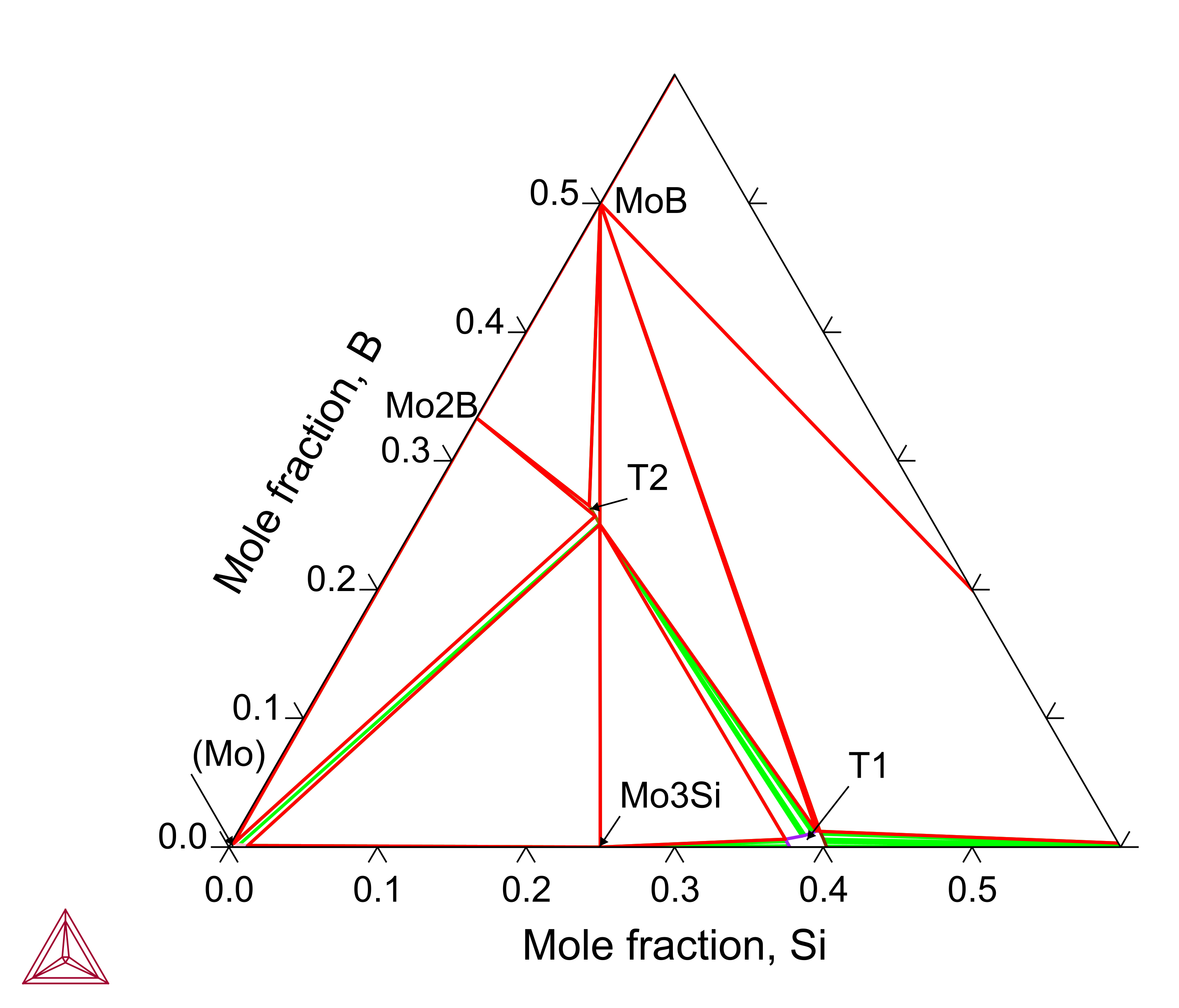
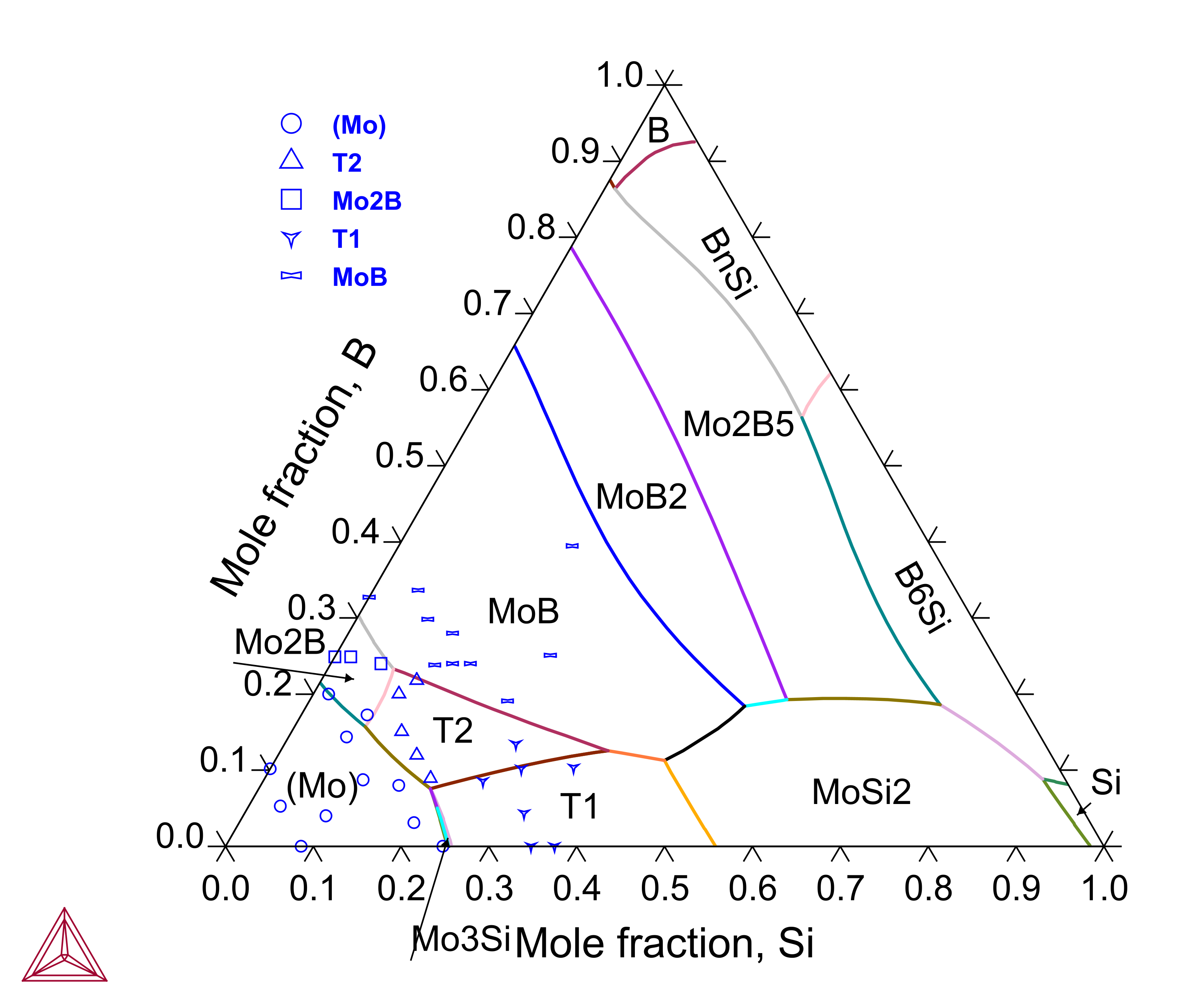
B-Mo-Si is the base system for Mo-based alloys frequently investigated in the literature, where the T2 (Mo5SiB2) phase forms and the T1 (Mo5Si3) phase dissolves a certain amount of boron. The B-Mo-Si ternary system has been assessed to also consider the experimentally observed primary solidification phases.
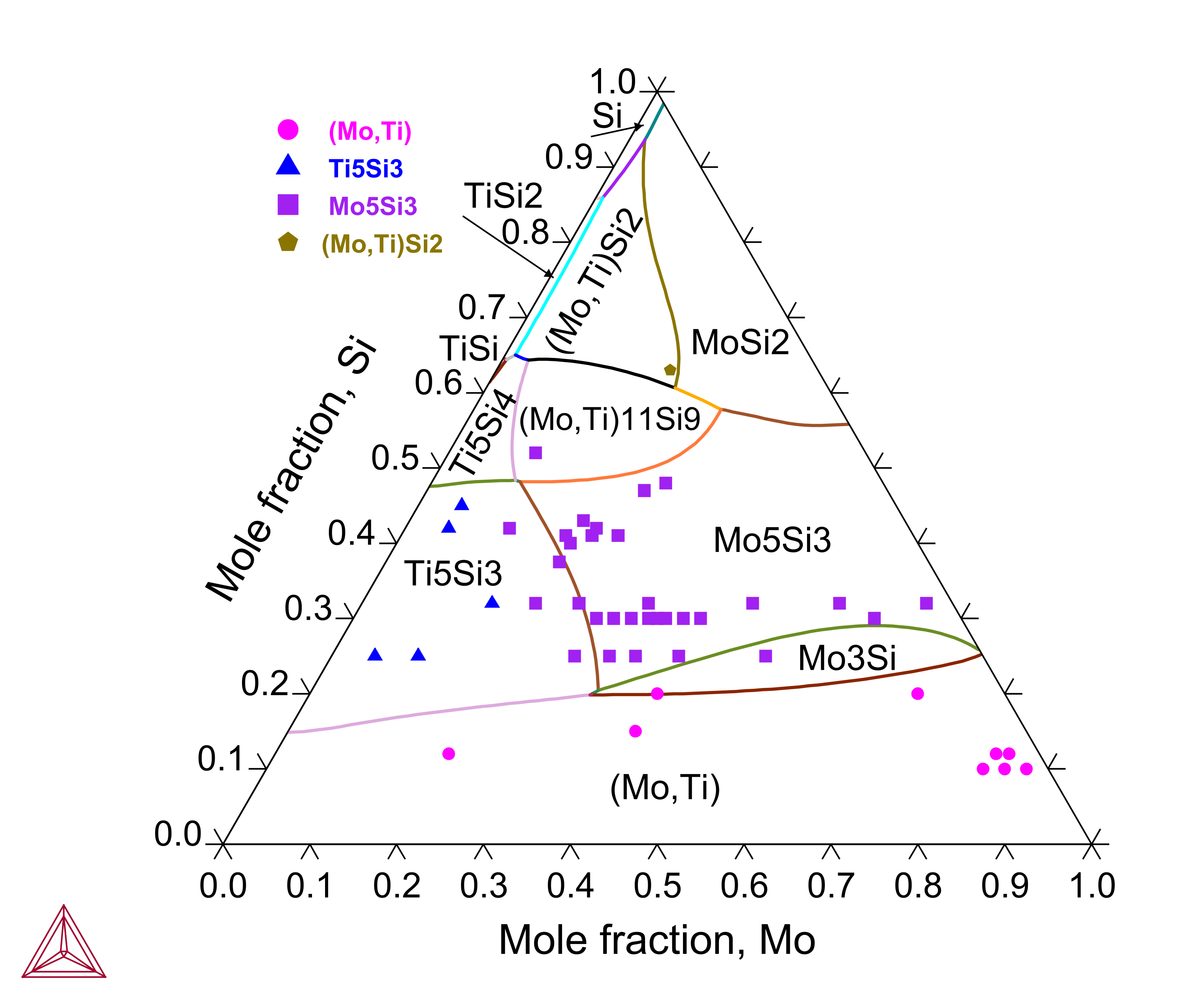
Mo-Si-Ti: Isothermal Sections and Liquidus Projection
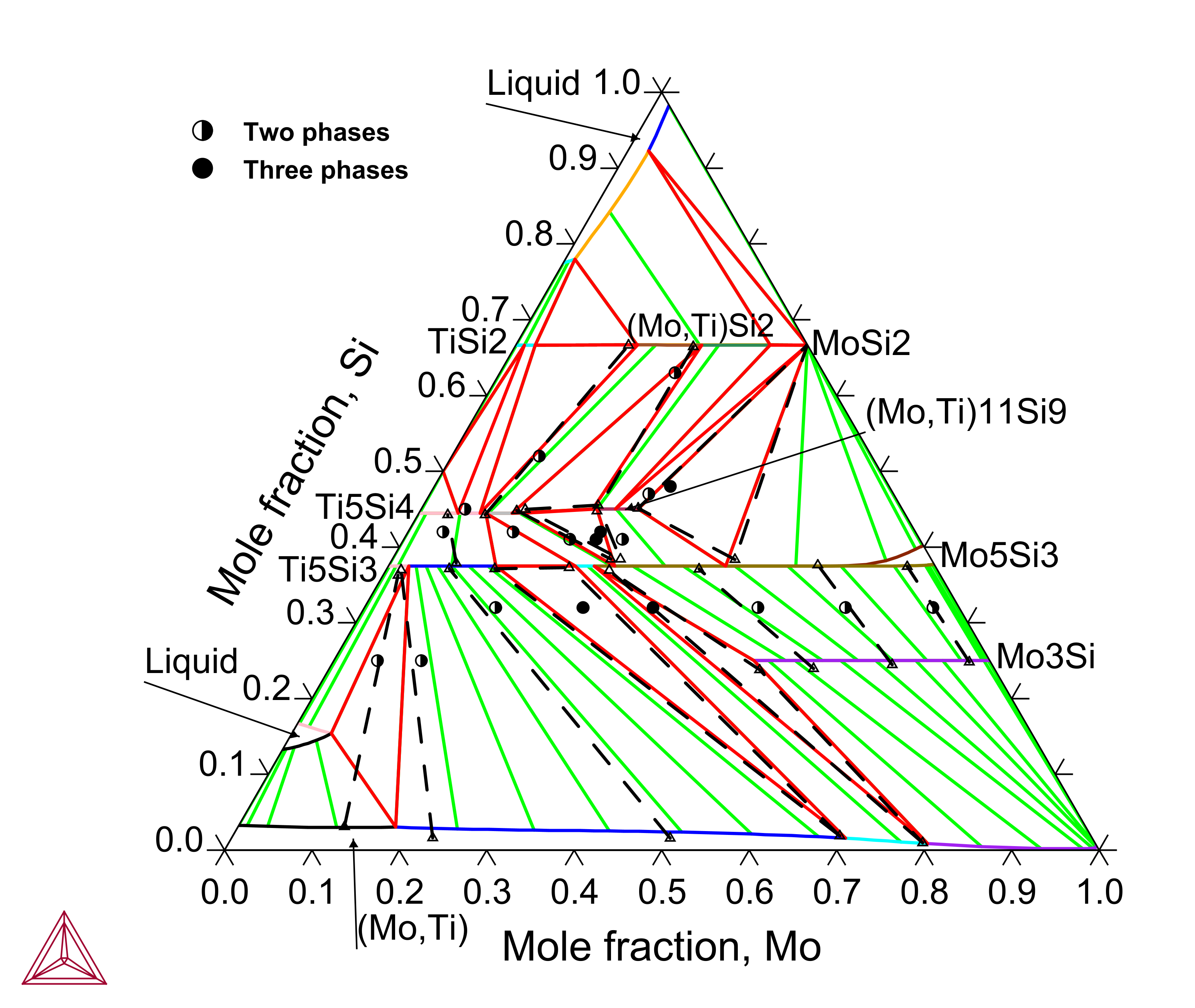
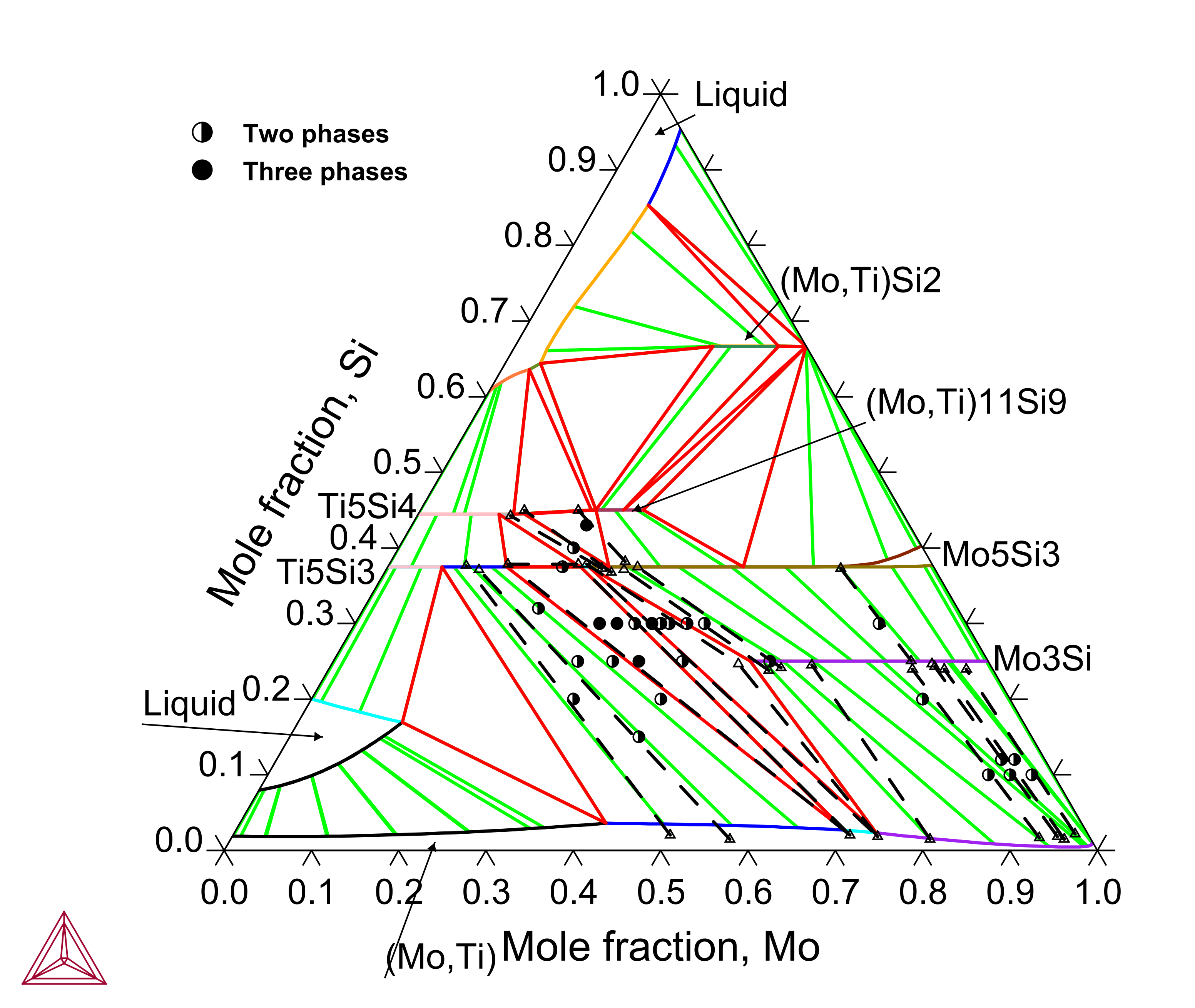
Titanium (Ti) is an important alloying element in the Mo-Si-B based alloys since Ti exhibits a large solubility in the Mo3Si and Mo5Si3 phases and the Ti5Si3 phase plays a critical role in tuning the alloys’ properties. The Mo-Si-Ti ternary system has been assessed to reproduce the experimental isothermal sections and liquidus projection.
Figure 3: Calculated Mo-Si-Ti isothermal section at 1425 °C compared with experimental data from [2003Yan]. The dashed lines represent experimentally measured tie-lines or tie-triangles.
Figure 4: Calculated Mo-Si-Ti isothermal section at 1600 °C compared with experimental data from [2003Yan]. The dashed lines represent experimentally measured tie-lines or tie-triangles.
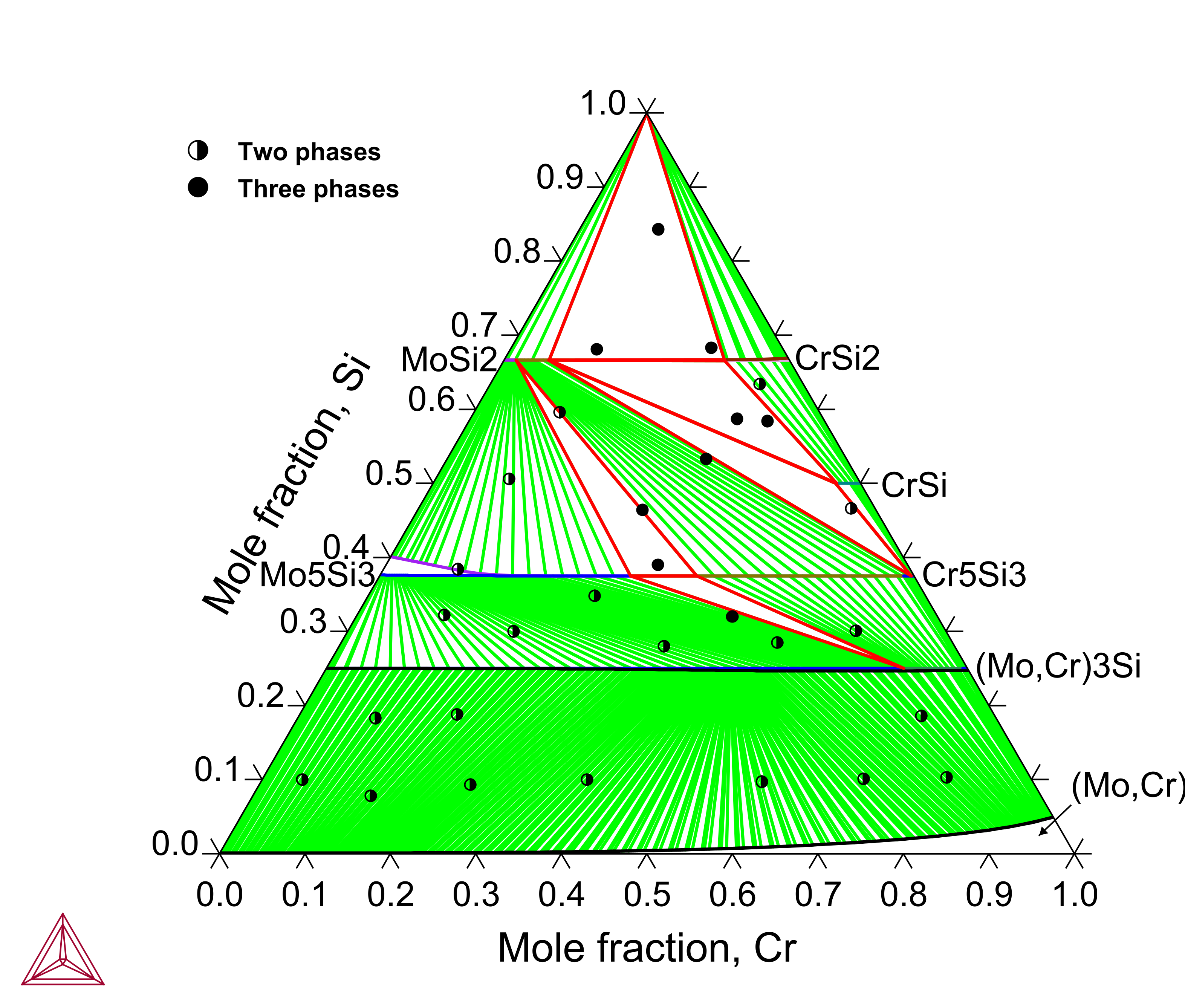
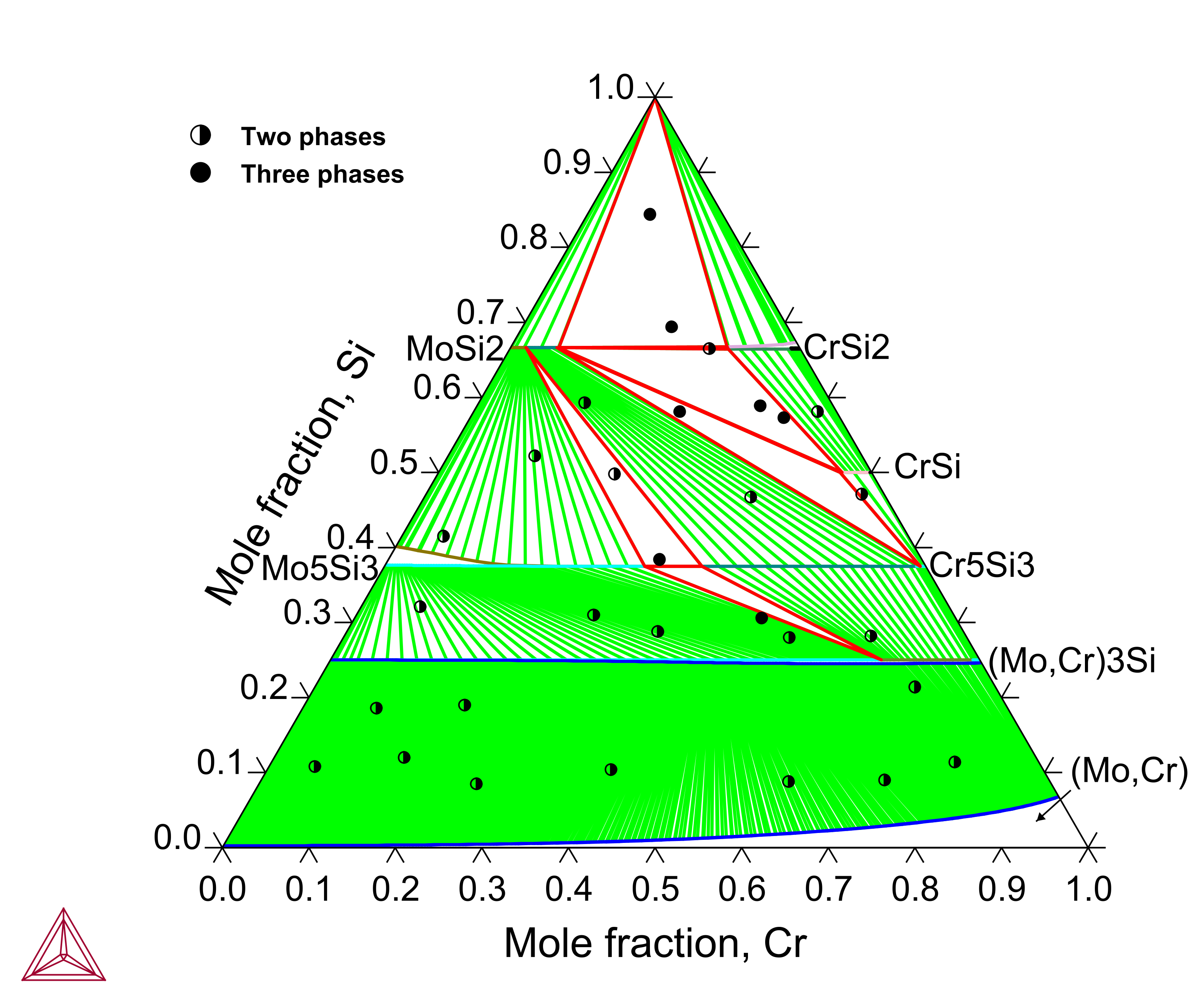
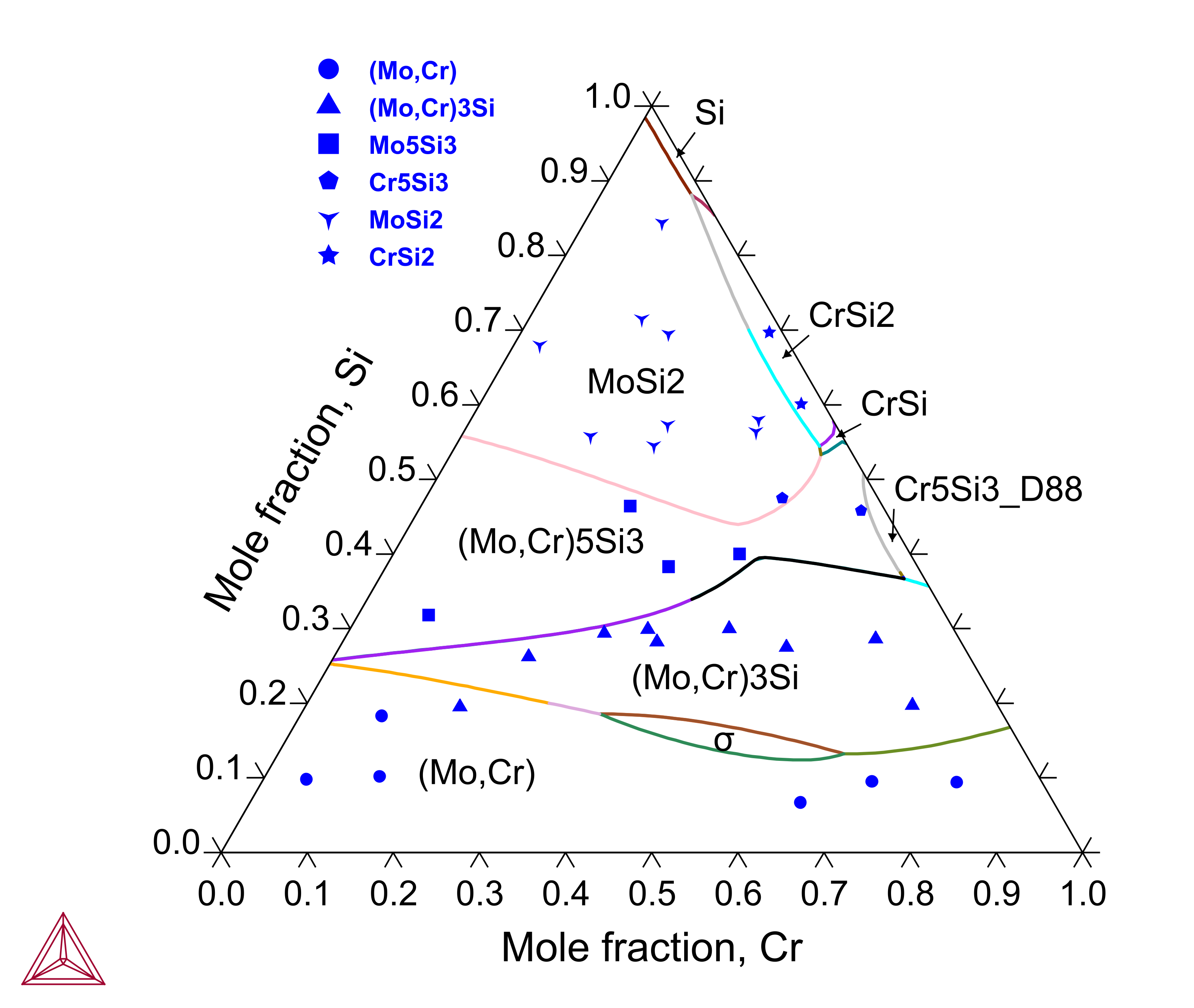
Cr-Mo-Si: Isothermal Sections and Liquidus Projection
Chromium (Cr) is another important alloying element in the Mo-Si-B based alloys where the low-temperature Cr5Si3 phase shares the same structure as Mo5Si3 and a continuous series of solid solution exists between Mo3Si and Cr3Si. The Cr-Mo-Si ternary system has been assessed to reproduce the experimental isothermal sections and liquidus projection.
Figure 6: Calculated Cr-Mo-Si isothermal section at 1000 °C compared with experimental data from [2022Wu].
Figure 7: Calculated Cr-Mo-Si isothermal section at 1200 °C compared with experimental data from [2022Wu].
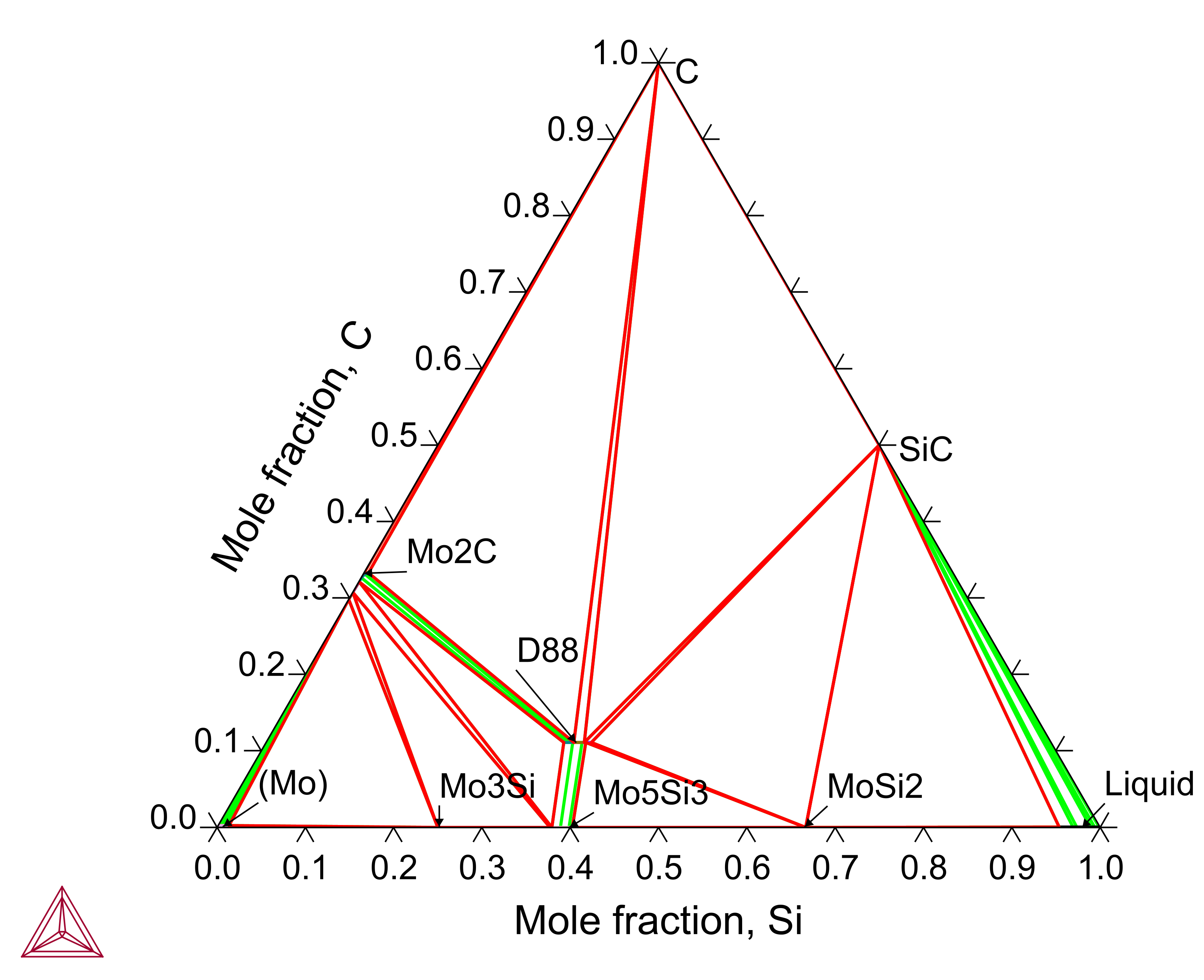
C-Mo-Si: Isothermal Section
Carbon (C) can be added to the Mo-Si-B based alloys to introduce desired carbide phases. Moreover, the introduction of C always leads to the formation of the D88 phase in the M-Si-C (M: Metal element) ternary systems, for example, in C-Mo-Si.
References
[2003Yan] Y. Yang, Y. A. Chang, L. Tan, Y. Du, Experimental investigation and thermodynamic descriptions of the Mo–Si–Ti system. Mater. Sci. Eng. A. 361, 281–293 (2003).
[2005Yan] Y. Yang, Y. A. Chang, Thermodynamic modeling of the Mo–Si–B system. Intermetallics. 13, 121–128 (2005).
[2022Wu] H. Wu, C. Li, C. Guo, Z. Du, Experimental determination of the isothermal sections and the liquidus surface projection of the Mo-Si-Cr ternary system. J. Alloys Compd. 922, 166164 (2022).