Solidification and Homogenization in the Diffusion Module (DICTRA)
The following examples are predicted using the Diffusion Module (DICTRA), with a combination of TCS Noble Metal Alloys Database (TCNOBL) and TCS Noble Metal Alloys Mobility Database (MOBNOBL).
Read more about the Diffusion Module (DICTRA) on our website. There is also a Getting Started with the Diffusion Module (DICTRA) page available. If you are in Thermo‑Calc, press F1 to search the help to learn about the available settings included with the Add-on Module.
Homogenization of Alloys
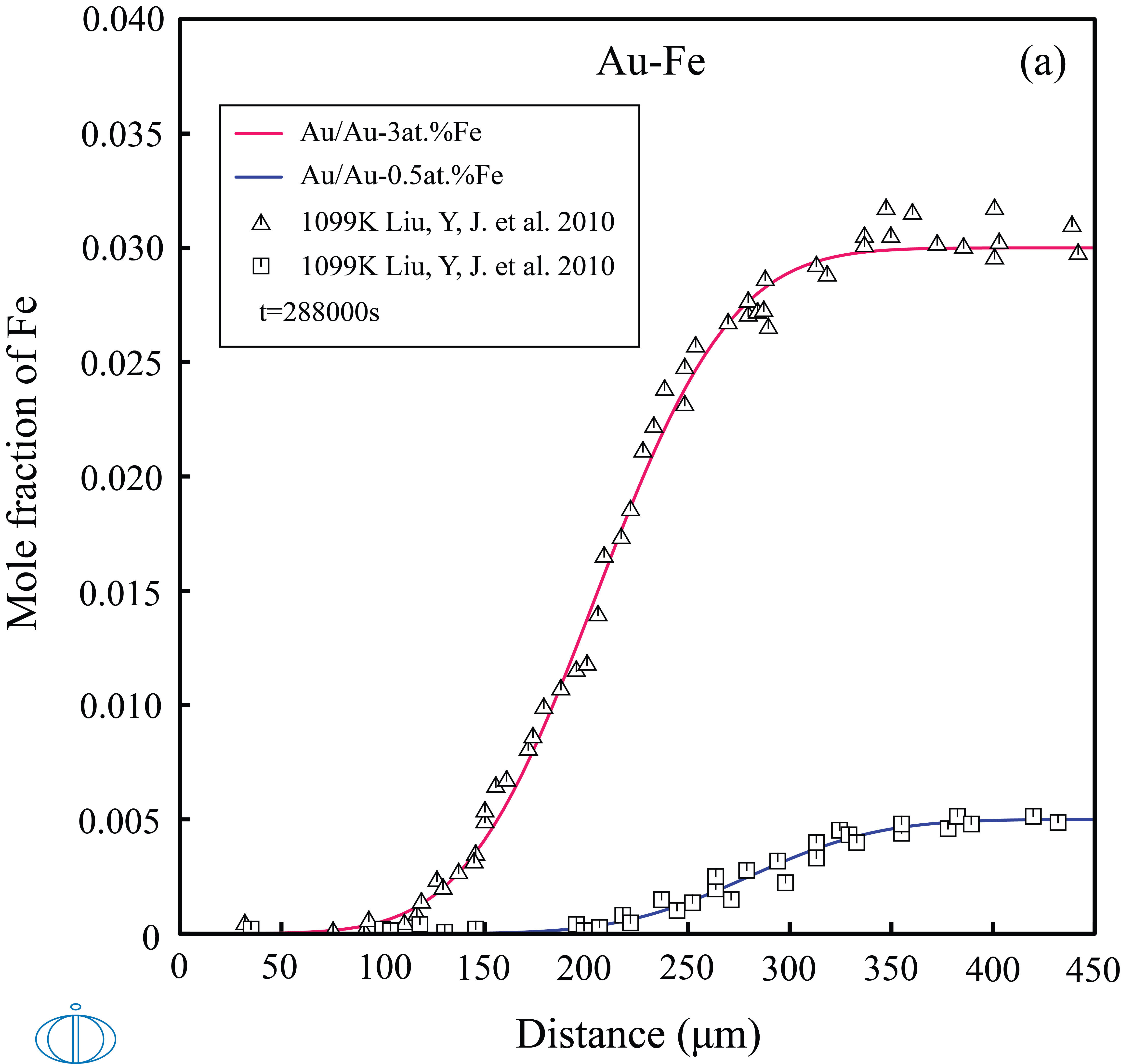
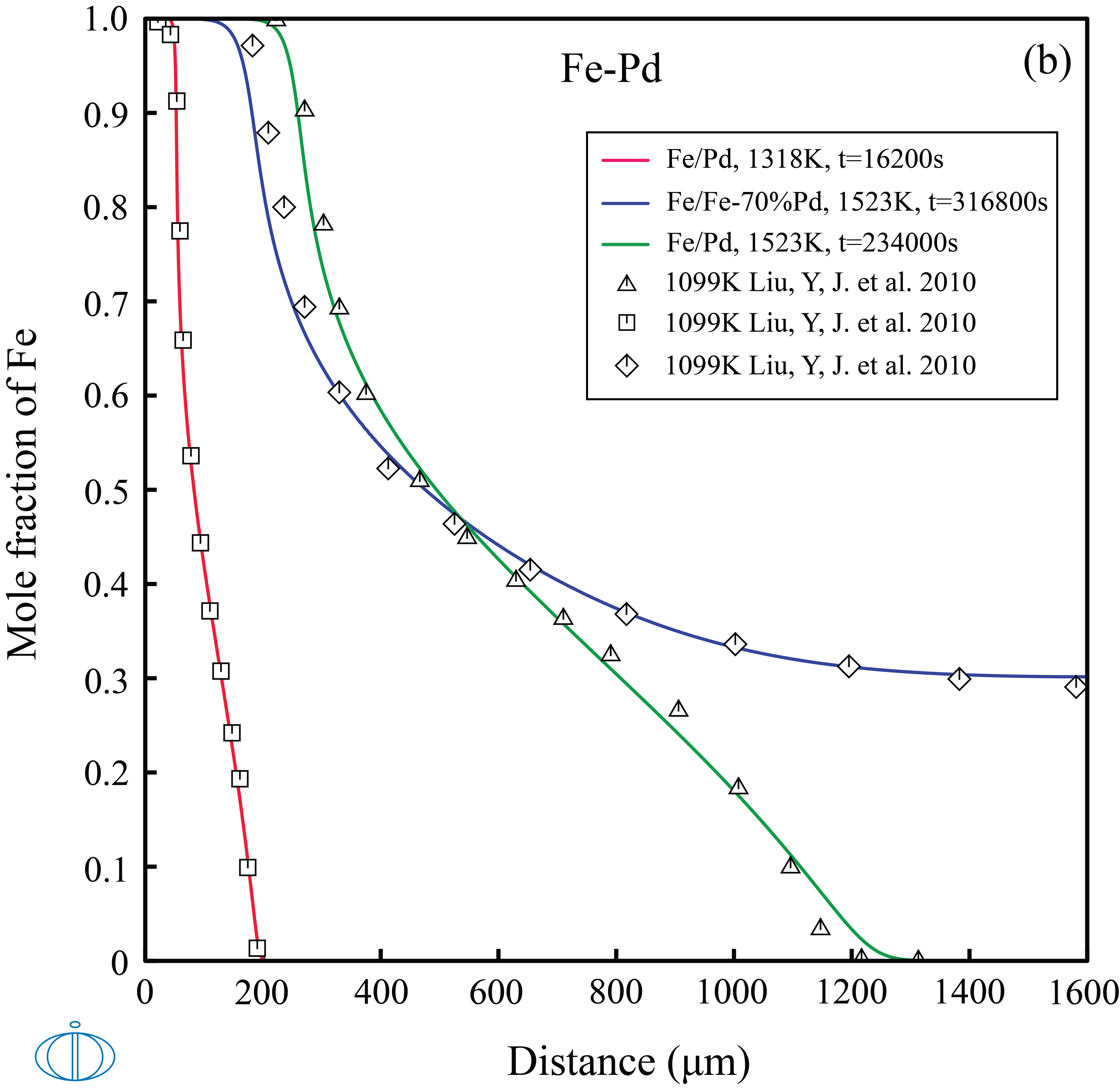
In this example, the concentration profiles in Au/Au-Fe and Fe/Pd-Fe diffusion couples at different times and temperatures.
Figure 1: The calculated concentration profiles in the (top labeled A) Au/Au-Fe and (bottom labeled B) Fe/Fe-Pd diffusion couples as a function of time and temperatures, compared with experimental data [2010Liu].
Simulation of Isothermal Solidification
Similar to other alloys, solidification is also one of the most critical processes in noble metal alloys. Being able to accurately simulate the solidification behaviors of a noble metal alloy is of great importance.
With solidification simulations, the evolution of phase fraction, the position of phase boundary, and so forth as a function of time and temperature can be predicted .
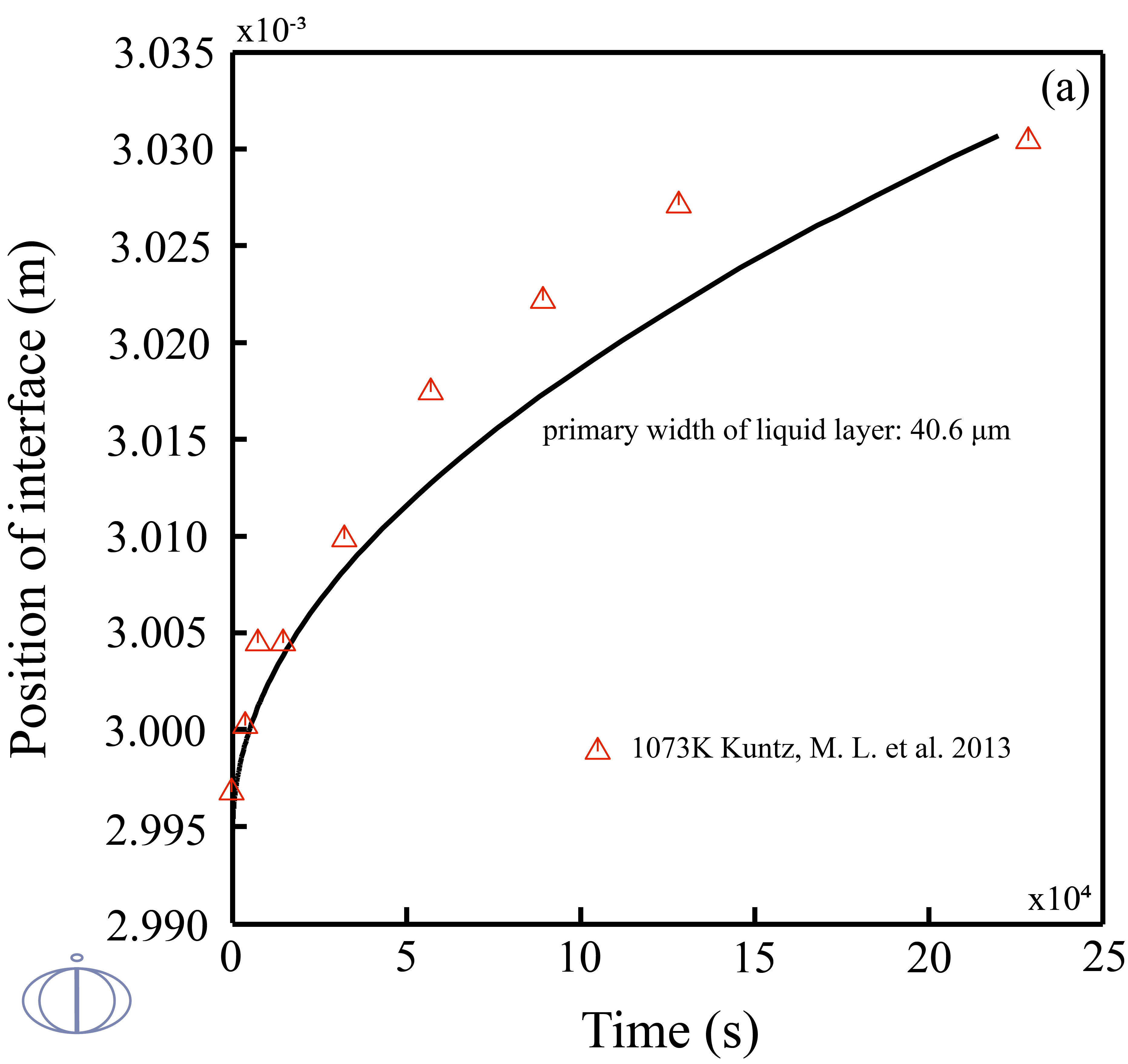
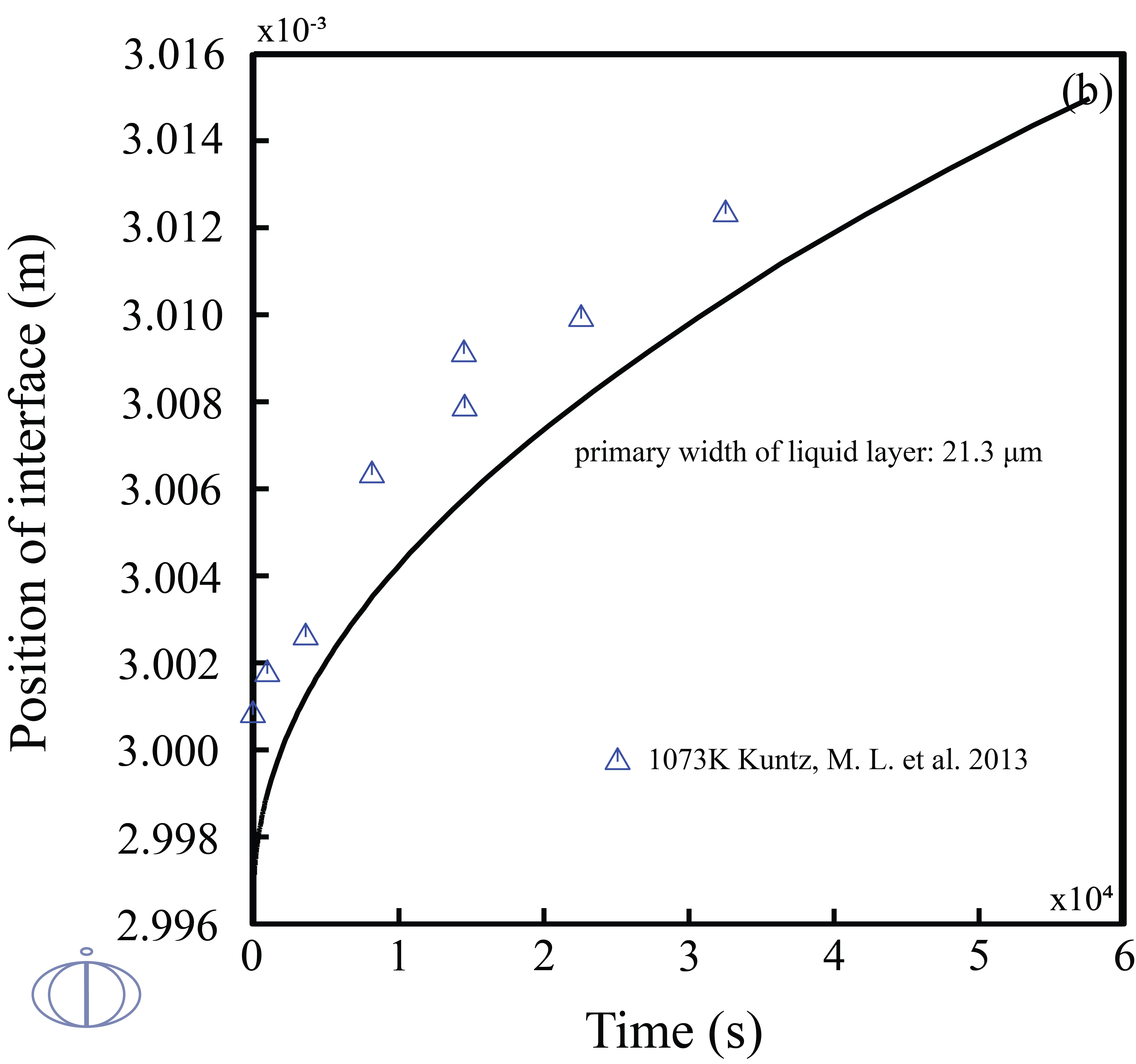
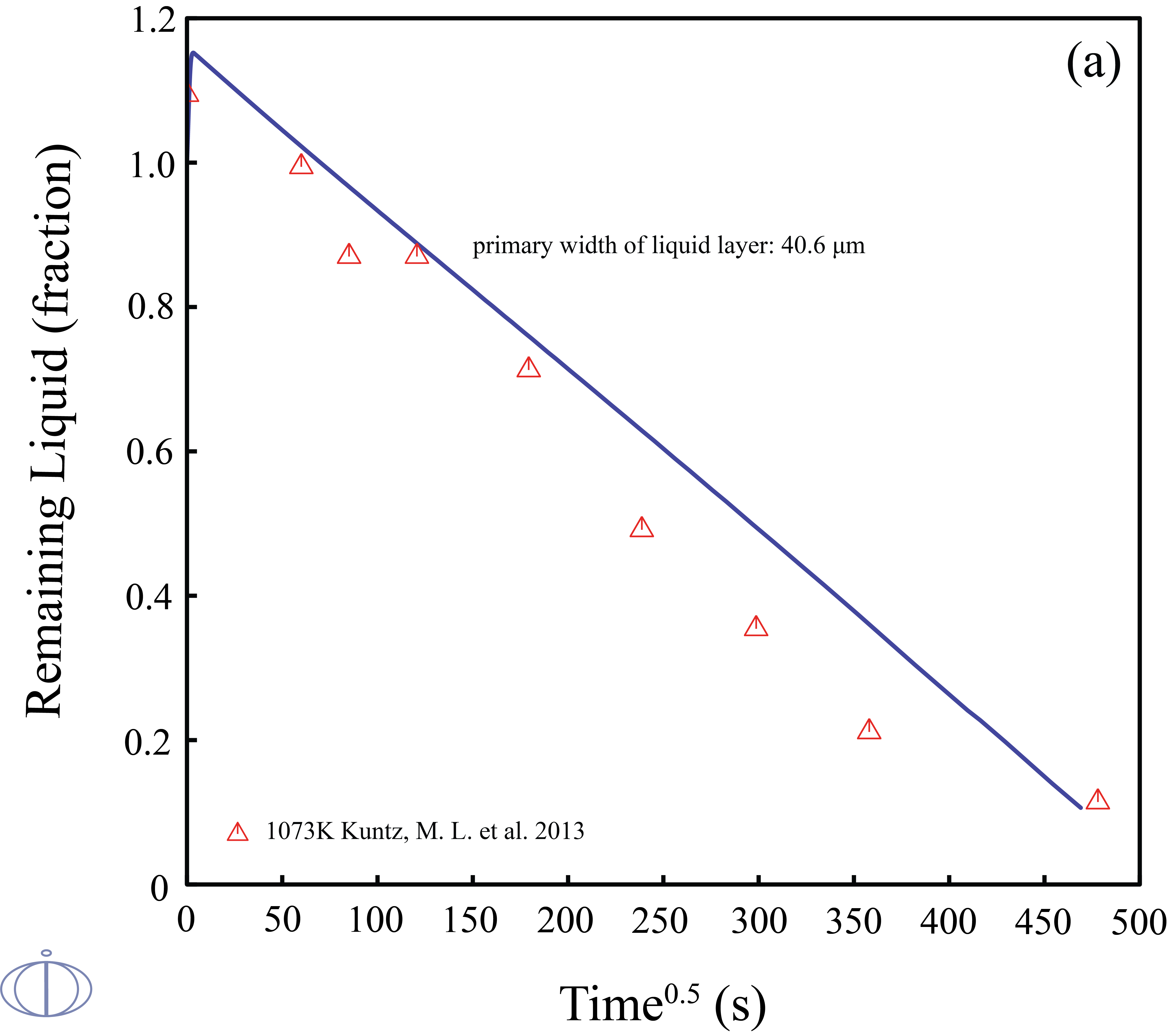
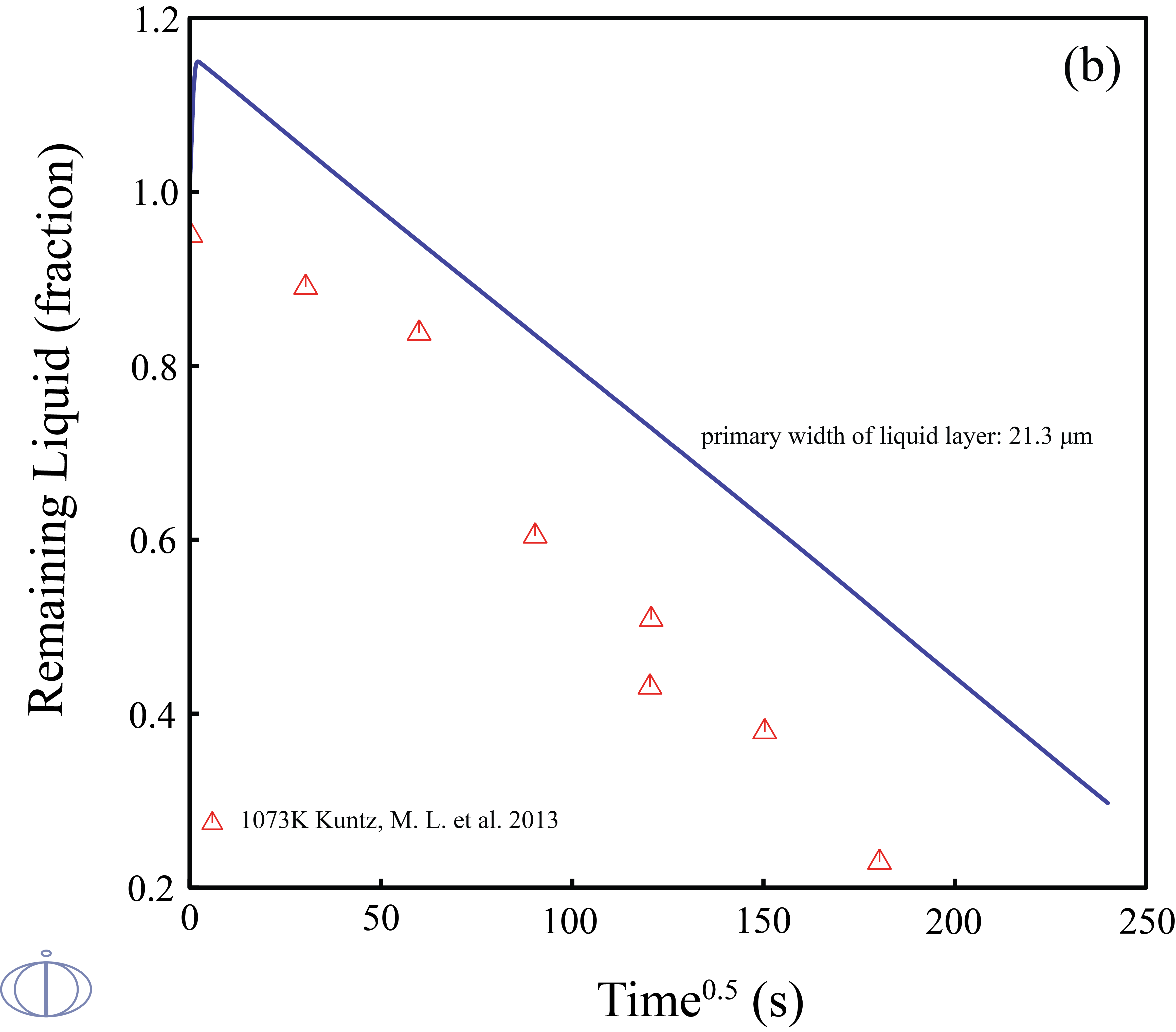
Transient liquid phase (TLP) bonding or diffusion brazing is a bonding process that has been developed to join materials which are otherwise very difficult to join. The bonding process is a brazing or soldering variation that utilizes an interlayer that melts at a temperature lower than that of the substrate to form a liquid at the faying surface. Due to the facts that 1) the TLP bonding is controlled by the diffusion of the solutes into the solid solvent that takes time on the order of hours; 2) designing a TLP process is time-consuming, and the examining results of estimating the width of the remaining eutectic liquid from the solidified joint cross sections may easily remain errors. Therefore, quantitatively and accurately predict the solid/liquid interface kinetics during the isothermal solidification stage of TLP bonding in an noble metal alloy is of great importance.
In this example, the phase transitions of Ag-43at.%Cu-14at.%Au during the isothermal solidification was predicted. The width of the liquid phase layer starts to increase in the beginning due to the change in solidus/liquidus composition and mass balance requirements, once the maximum liquid width has been reached, the solutes will diffuse into the base metal and the fraction of liquid phase starts to decrease.
Figure 2: The calculated positions of interface vs. time at 1073K in the diffusion couples of the (solid) pure Ag/ (liquid)Ag-43at.%Cu-14at.%Au with the primary widths of liquid layer (top labeled A) 40.6 μm and (bottom labeled B) 21.3 μm along with the experimental data [2013Kun].
Figure 3: The calculated fraction of remaining liquid vs. time at 1073K in the diffusion couples of the (solid) pure Ag/ (liquid)Ag-43at.%Cu-14at.%Au with the primary widths of liquid layer (top labeled A) 40.6 μm and (bottom labeled B) 21.3 μm along with the experimental data [2013Kun].
References
[2010Liu] Y. Liu, J. Wang, Y. Du, L. Zhang, D. Liang, Mobilities and diffusivities in fcc Fe–X (X=Ag, Au, Cu, Pd and Pt) alloys. Calphad. 34, 253–262 (2010).
[2013Kun] M. L. Kuntz, B. Panton, S. Wasiur-Rahman, Y. Zhou, S. F. Corbin, An Experimental Study of Transient Liquid Phase Bonding of the Ternary Ag-Au-Cu System Using Differential Scanning Calorimetry. Metall. Mater. Trans. A. 44, 3708–3720 (2013).