Hydrogen Impact on Phase Equilibria and Transition Temperatures
Hydrogen is incorporated in the TCS Zr-based Alloys Database (TCZR) where the hydrogen solubility and hydrides in binary systems and ternary Zr-Nb-H are carefully assessed. In reality, oxidation of zirconium by water releases hydrogen gas, which partly diffuses into the alloy.
H Solubility in HCP_Zr and Zr-H Phase Diagram
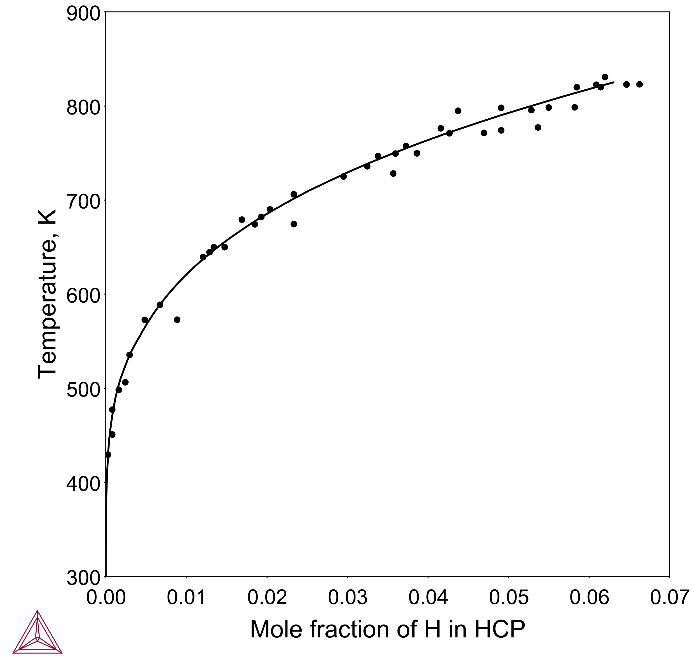
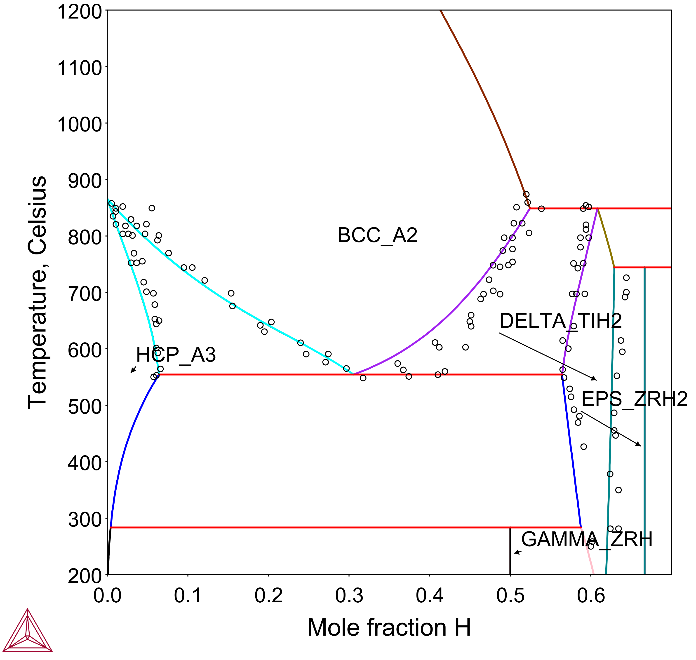
In this example, Figure 1 shows the calculated H solubility in HCP_Zr and Figure 2 shows the calculated Zr-H phase diagram with types of hydrides.
Hydrogen in Zr alloys decreases α/β transus temperatures [2008Zha] and may form zirconium hydrides. The hydrides are less dense and are mechanically weaker than the alloy. The formation results in blistering and cracking of the cladding—a phenomenon known as hydrogen embrittlement.
Zr-H-Nb Isotherms
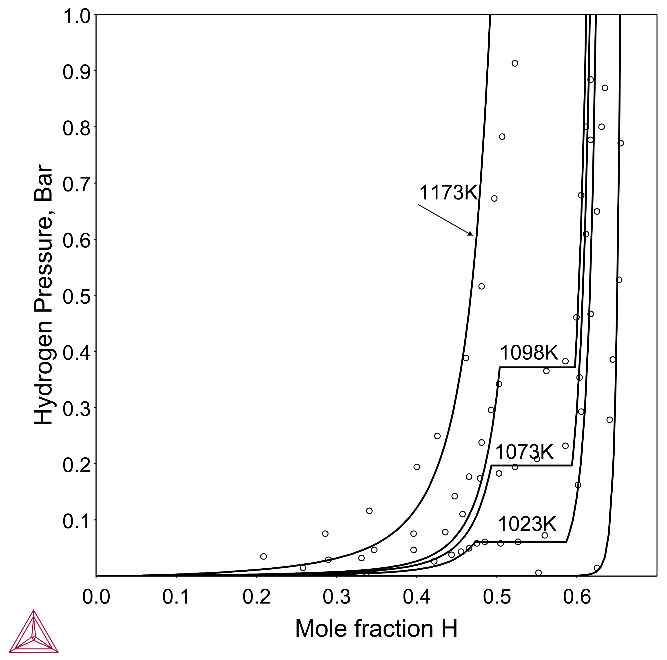
Figure 3 shows the calculated pressure-composition isotherms of the Zr–H–Nb system (wt.%Nb = 2.5) at different temperatures with experimental data from Sinha and Singh [1972Sin]. In the isotherms, where the pressure is composition-dependent, a single solid phase exists. On the plateaus, where the pressure is independent of composition, two solid hydride phases are in equilibrium. The phase boundary composition data, hydrogen equilibrium pressure for the two-phase region and the isobaric limit can be defined from the isotherms. In general, the agreement between calculated and experimental data is satisfactory. As the temperature decreases, the hydrogen composition increases and the equilibrium phases of the system also change.
Figure 3: Calculated pressure-composition isotherms of Zr-2.5 wt.%Nb-H with experimental data from [1972Sin].
References
[1972Sin] V. K. Sinha, K. P. Singh, A pressure-composition-temperature study of Zr-Nb-H system. Metall. Trans. 3, 1581–1585 (1972).
[1990Zuz] E. Zuzek, J. P. Abriata, A. San-Martin, F. D. Manchester, The H-Zr (hydrogen-zirconium) system. Bull. Alloy Phase Diagrams. 11, 385–395 (1990).
[2008Zha] W. Zhao, Y. Liu, H. Jiang, Q. Peng, Effect of heat treatment and Nb and H contents on the phase transformation of N18 and N36 zirconium alloys. J. Alloys Compd. 462, 103–108 (2008).