Oxygen Impact on Phase Equilibria and Transition Temperatures
Oxygen is one of the major impurities in Zr-based alloys. The oxygen level in Zr alloys inevitably accounts for various features such as α/β transus temperatures, phase compositions, and for fractions vs. temperature. In the TCS Zr-based Alloys Database (TCZR) the oxygen solubility and oxide phases in binary and ternary Zr systems are carefully assessed.
Zr-Ni-O
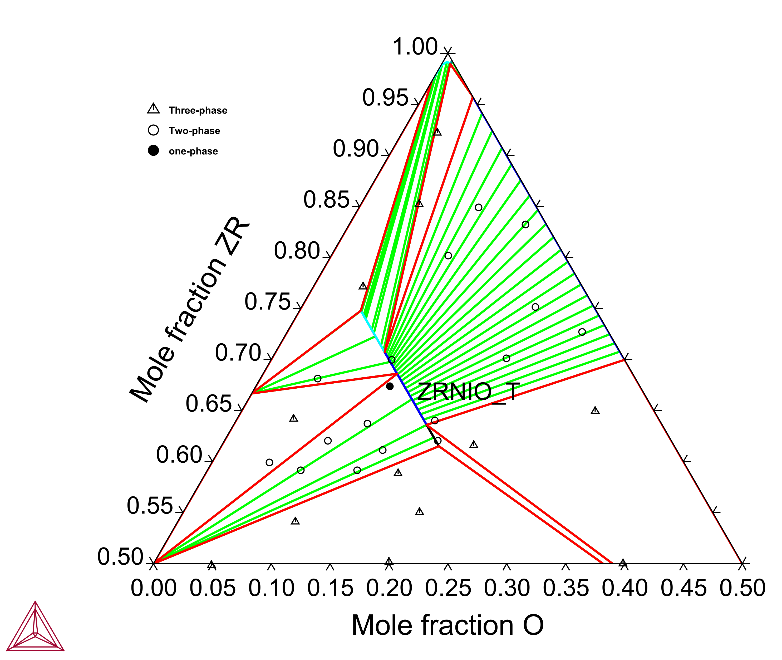
The plot below shows the calculated Zr-rich side of the Zr‑Ni-O system. A ternary ZrNiO_T phase occurs in an elongated phase field where O concentration varies between about 6 and 19 at.%.
α+β → β Transition Temperatures
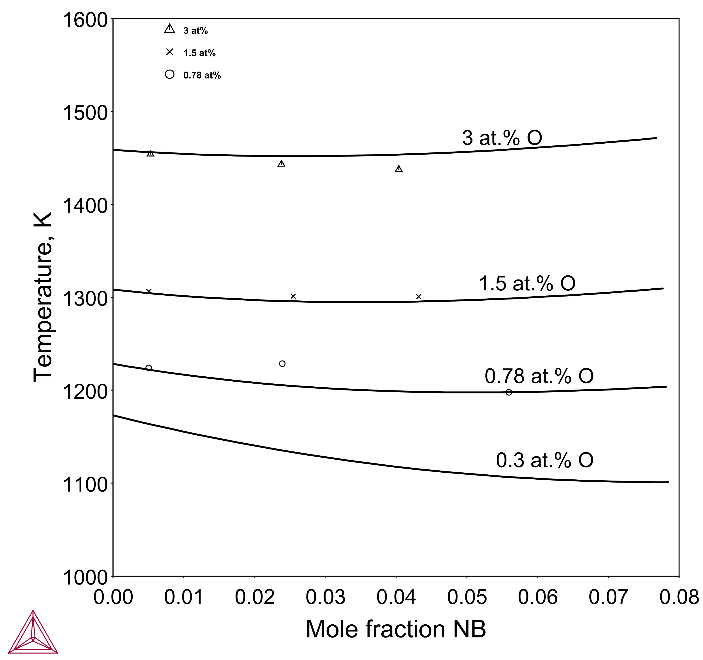
The plot below shows the calculated versus measured α+β → β transition temperatures at different oxygen levels in the Zr-rich region. The calculation results are well in agreement with the experimental ones.
Figure 2: Calculated versus measured α+β → β transition temperature in the Zr-rich region with experimental data from [1971Hun].
References
[1961Nev] M. V. Nevitt, J. W. Downey, The Zirconium-Rich Corners of the Ternary Systems Zr-Co-O and Zr-Ni-O, Trans. Met. Soc. AIME, 221 (1961) 1014-1017.
[1971Hun] C. E. L. Hunt, P. Niessen, The continuous cooling transformation behaviour of zirconium-niobium-oxygen alloys. J. Nucl. Mater. 38, 17–25 (1971).