Moving Boundary Examples
Austenite (γ) to ferrite (α) transformation in a binary Fe-C alloy
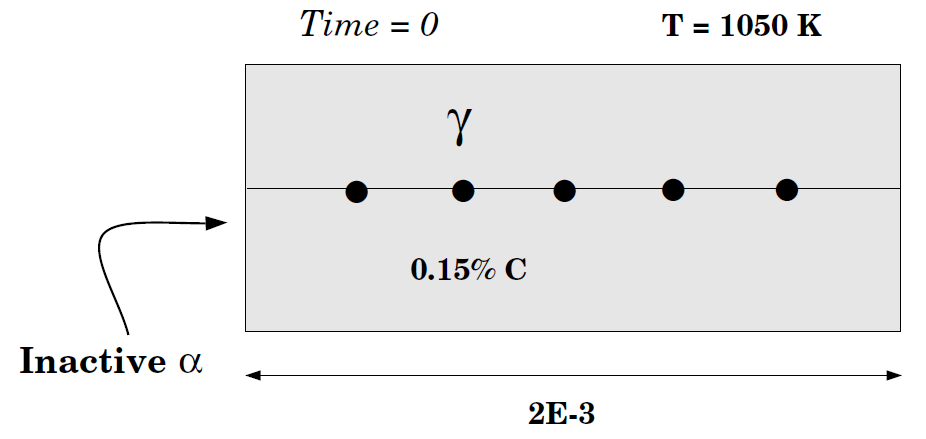
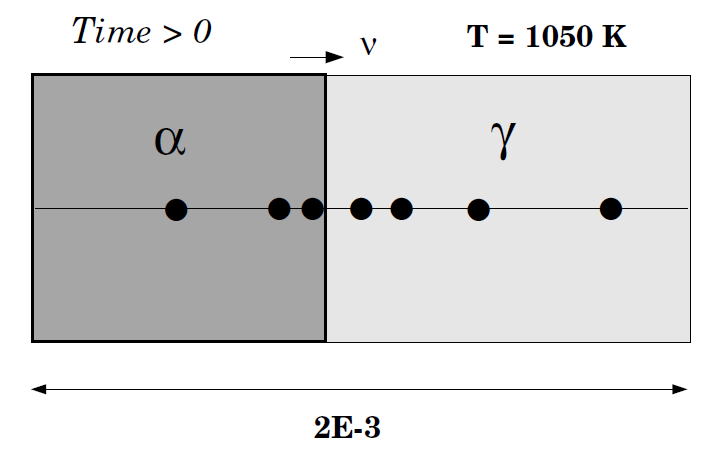
This example calculates a ferrite (BCC)/austenite (FCC) transformation in a binary Fe-C alloy. The initial state is an austenite of 2 mm thickness. The composition of the austenite is Fe-0.15wt%C. After austenitization the specimen is quenched down to 1050K. The system is assumed closed, so no boundary conditions are set (a closed system is the default). Ferrite is expected to grow into the austenite. For this reason you start with a thin region with ferrite adjacent to the austenite.
Austenite (γ) to ferrite (α) transformation in a binary Fe-C alloy: Gradual cool down to 1050K
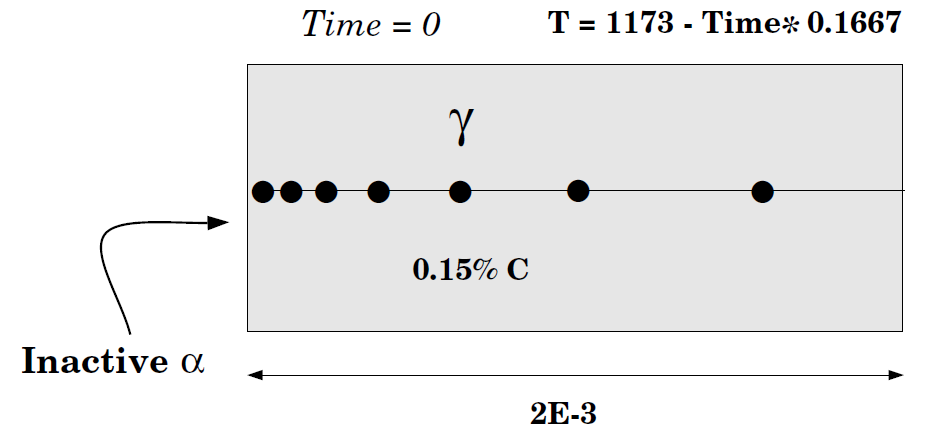
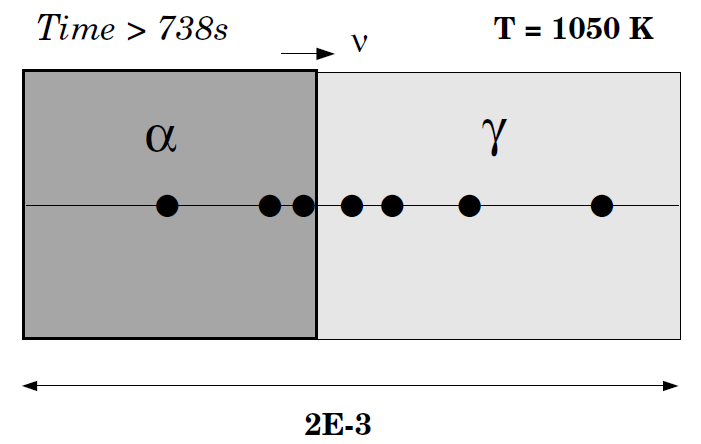
This is the same example as in exb1a and exb1b but now the simulation starts at a higher temperature and assumes a gradual cooling down to 1050 K.
When 1050 K is reached, the temperature is kept constant and thus has an isothermal transformation. As in exb1b, ferrite is in an inactive phase adjacent to the initial austenite.
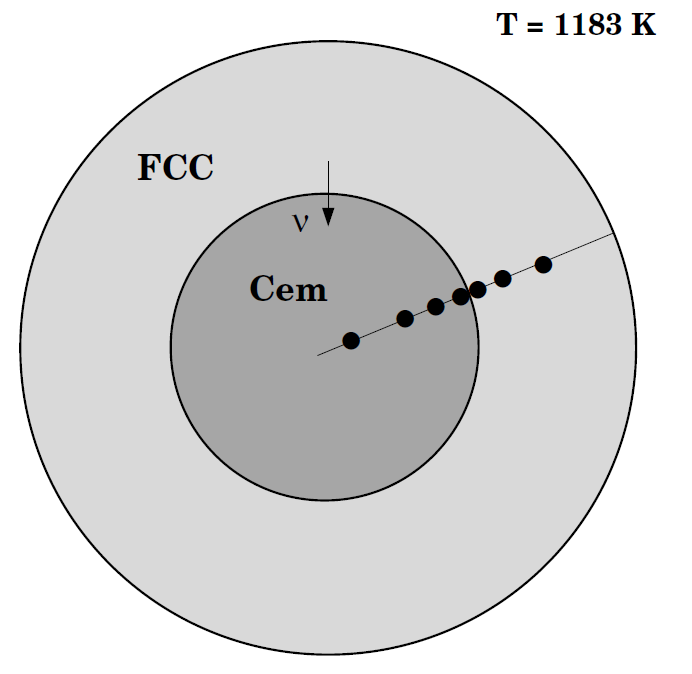
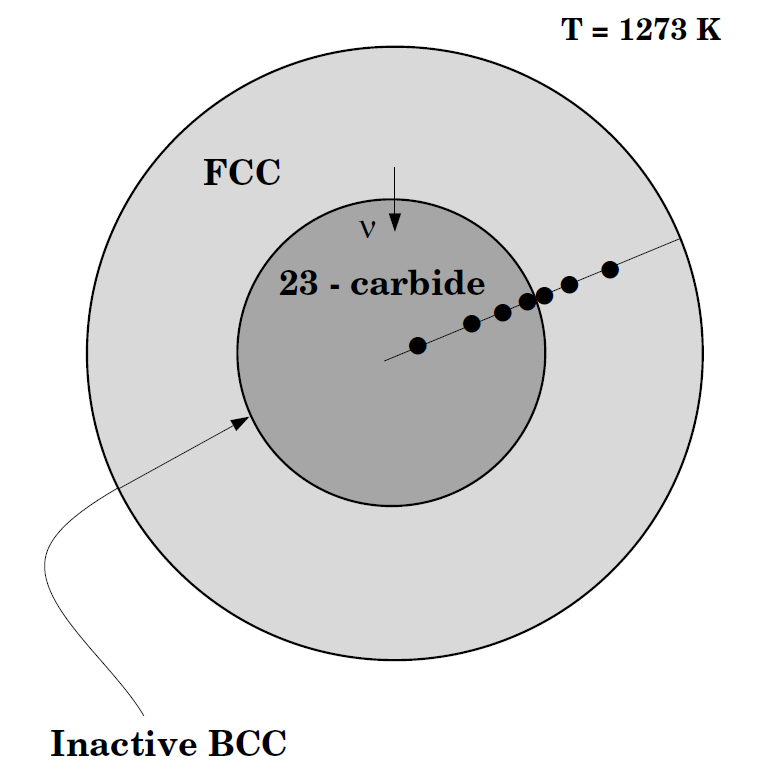
Cementite dissolution in an Fe-Cr-C alloy
This example calculates the dissolution of a spherical cementite particle in an austenite matrix. This case is from [1991Liu].
In order to achieve the correct average composition in the calculation it is necessary to take into account the fact that the calculation is set up using the volume fraction of the phases. To calculate the initial state at the heat treatment temperature we need first to determine the state at the normalizing temperature. To calculate the volume fraction of the phases we need to enter a number of functions that calculate these quantities.
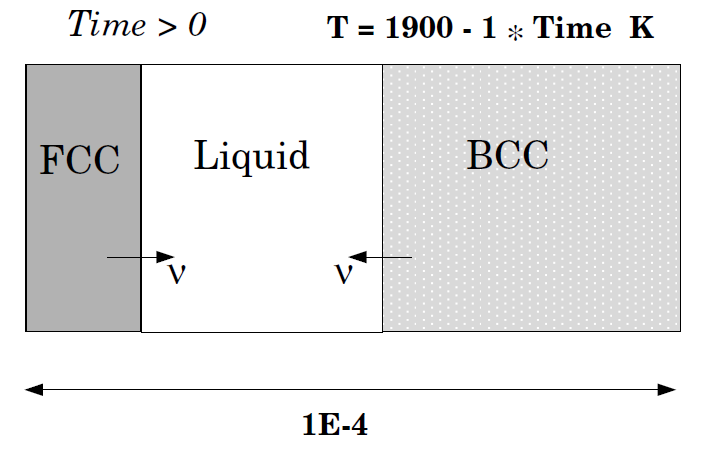
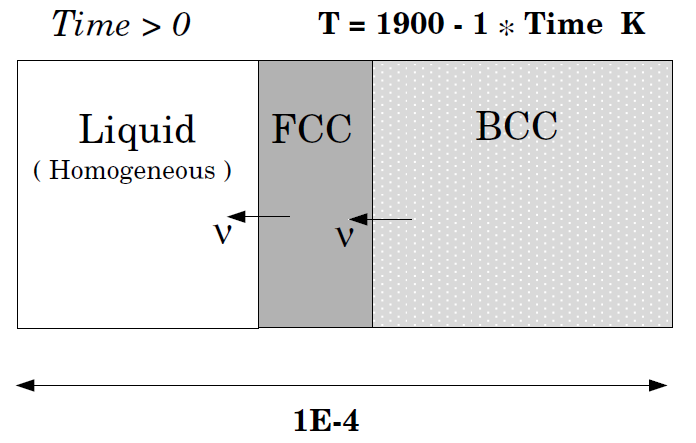
Solidification path of an Fe-18%Cr-8%Ni alloy: Eutectic reaction
This example demonstrates the solidification path of an Fe-18%Cr-8%Ni alloy. A eutectic reaction is assumed, LIQUID -> BCC + FCC. Hence the BCC and FCC regions should be on separate sides of the liquid region. Comparison is made with both a Scheil-Gulliver simulation and equilibrium solidification conditions, both done in Thermo‑Calc.
This example uses the TCFE and MOBFE databases. Licenses are required to run the example.
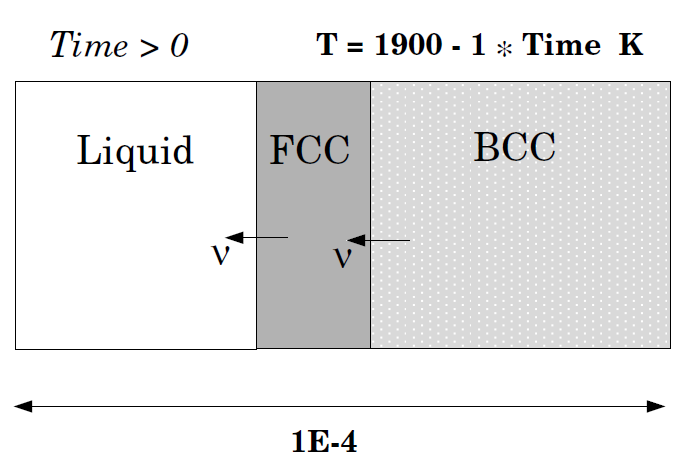
Solidification path of an Fe-18%Cr-8%Ni alloy: Peritectic reaction
This example is the same as exb4a but now a peritectic reaction is assumed: LIQUID + BCC -> FCC. Hence the FCC region should appear in between the LIQUID and the BCC. Comparison is made with both a Scheil-Gulliver simulation and equilibrium solidification conditions, both done in Thermo‑Calc.
Solidification path of an Fe-18%Cr-8%Ni alloy: Peritectic reaction, homogeneous liquid
This example is the same as exb4b but now the diffusivity data is amended for the LIQUID and a very high value for the diffusivity is used in order to simulate a case where we assume that the composition in the LIQUID is always homogeneous. This case should be considered less realistic than exb4b. Comparison is made with both a Scheil-Gulliver simulation and equilibrium solidification conditions, both done in Thermo‑Calc.
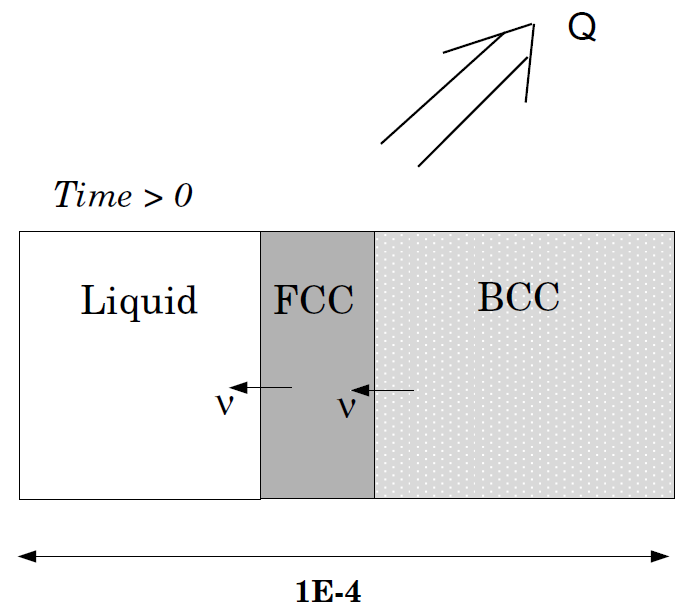
Solidification path of an Fe-18%Cr-8%Ni alloy: Peritectic reaction, heat-flux controls the temperature
This example is the same as exb4b but instead of controlling the temperature the amount of heat extracted is given. Comparison is made with both a Scheil-Gulliver simulation and equilibrium solidification conditions, both done in Thermo‑Calc.
This example uses the FEDEMO and MOBFE2 database. A license is required for the MOBFE database to run the example.
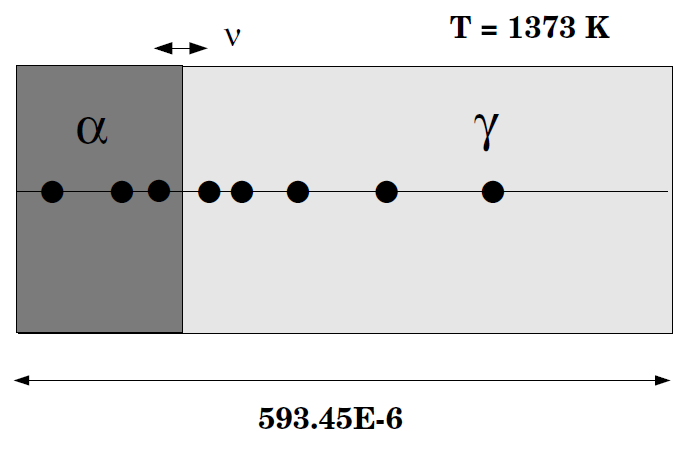
γ/α/γ Diffusion couple of Fe-Ni-Cr alloys
This example demonstrates the evaluation of a ternary Fe-Cr-Ni diffusion couple. A thin slice of α phase (38%Cr,0%Ni) is clamped between two thicker slices of γ phase (27%Cr, 20%Ni). The assembly is subsequently heat treated at 1373K.
This set up corresponds to diffusion couple in [1993Kaj-1]. Also see [1993Kaj-2].
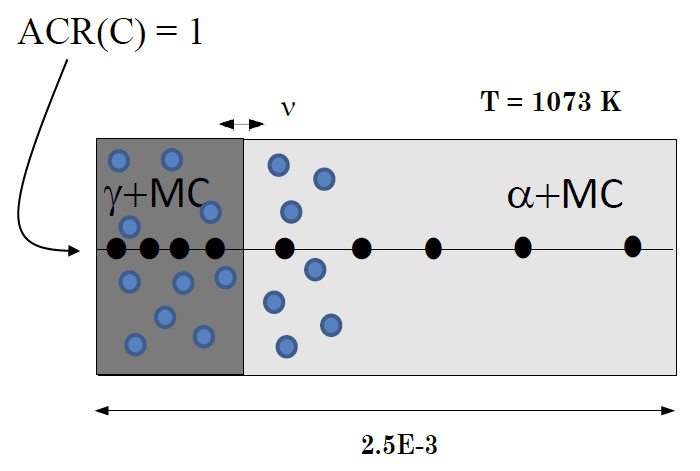
Moving boundary problem with multiple phases on each side of the boundary
This example shows how to enter dispersed phases on either side of a phase interface. The particular case shows how the kinetics of a ferrite to austenite transformation is affected by simultaneous precipitation of niobium carbide. The transformation is caused by carburization.
This example uses the TCFE, SSUB and MOBFE databases. These licenses are required to run the example.
[1991Liu] Z.-K. Liu, L. Höglund, B. Jönsson, J. Ågren, An experimental and theoretical study of cementite dissolution in an Fe-Cr-C alloy. Metall. Trans. A 22, 1745–1752 (1991).
[1993Kaj-1] M. Kajihara, C.-B. Lim, M. Kikuchi, Experimental Study on Dissolution of ALPHA Phase in GAMMA/ALPHA/GAMMA Diffusion Couples of the Fe-Cr-Ni System. ISIJ Int. 33, 498–507 (1993).
[1993Kaj-2] M. Kajihara, M. Kikuchi, Numerical analysis of dissolution of α phase in γ/α/γ diffusion couples of the Fe-Cr-Ni system. Acta Metall. Mater. 41, 2045–2059 (1993).