Electrical Conductivity for Ionic Liquid Model
The model for the electrical conductivity of molten slags is mainly based on the theory of the electrical conductivity of ionic solutions. The total conductivity is the sum of the contributions from all cations including those network-forming cations modeled as neutrals in the two sublattice ionic liquid model, such as liquid silicon oxide. For slags containing transition metal ions of different oxidation states, the electronic contribution due to the electron or hole exchange between these ions is also considered.
In short, the electrical conductivity of molten slags consists of the following:
- Ionic conductions contributed by cations in the ionic liquid.
- Electronic conductions from the cations having more than one oxidation states (Fe2+- Fe3+, Mn2+- Mn3+ and so on).
The composition and temperature dependence of the total electrical conductivity of molten slags,  , is represented by:
, is represented by:
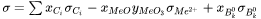
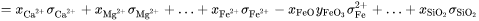
where 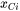 is the mole fraction of cation
is the mole fraction of cation 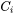 , and its electrical conductivity,
, and its electrical conductivity, 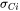 , is written in the form of the Nernst-Einstein equation as:
, is written in the form of the Nernst-Einstein equation as:
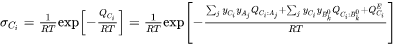
where 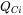 is the activation Gibbs energy, of which the composition dependence has been expanded in a CALPHAD fashion.
is the activation Gibbs energy, of which the composition dependence has been expanded in a CALPHAD fashion.
The model parameters are the activation Gibbs energy of end-members and excess activation Gibbs energy in the above equation. They can be entered in the GES module or TDB file by using the keyword ELQ, for example,
PARAM ELQ(IONIC_LIQ&Ca+2,CA+2:O-2;0)
PARAM ELQ(IONIC_LIQ&Ca+2,CA+2:SIO4-4;0)
PARAM ELQ(IONIC_LIQ&Ca+2,CA+2:SIO2;0)
All the quantities can be calculated, retrieved, shown and plot in Thermo‑Calc via the variables in the Console Mode or via the quantity names in the Graphic Mode.
The electrical conductivity and resistivity of molten slags modeled as the ionic liquid phase can be calculated usually through a single point or step equilibrium calculation in Console Mode. The results can be shown or plotted by using the variables ELCD(IONIC) and ELRS(IONIC), respectively.
On the Plot Renderer in Graphical Mode, the electrical conductivity and resistivity of molten slags modeled as the ionic liquid phase can be calculated by using the Single point or One Axis calculation type in Graphical Mode. The corresponding tabulation or plot variables are Electrical Conductivity and Electrical Resistivity, respectively.
With these Software Development Kits (SDKs), this property can be plotted using property-diagram_calculation() or with_batch_ equilibrium_calculation(). The plot variables are ELCD(IONIC) and ELRS(IONIC).
These can also be accessed in both ThermodynamicQuantity and ScheilQuantityclasses. You can, for example, use ThermodynamicQuantity.electrical_conductivity("IONIC") and ThermodynamicQuantity.electrical_resitivity("IONIC") for electrical conductivity and electrical resistivity, respectively.
You can also get the individual values e.g. from an equilibrium calculation and use in whatever way you want, e.g. feed into other models: result.get_value_of("ELCD(IONIC_LIQ)"), where 'result' is the result of one equilibrium calculation. It matches show_value in Console Mode.
This can also be written result.get_value_of(ThermodynamicQuantity.electrical_conductivity("IONIC_LIQ")) or result.get_value_of(ThermodynamicQuantity.electrical_resitivity("IONIC_LIQ")).