Tips for Analyzing Scheil Solidification Simulations
AZ91 is a series of the most important die casting Mg alloys. Using the TCS Mg-based Alloys Database (TCMG), you can simulate the solidification process of the AZ91 alloy using Scheil calculations.
Read more about Scheil Solidification Simulations on our website, including how to select the right model for your simulation. If you are in Thermo‑Calc, press F1 to search the help to learn about using Scheil.
The typical microstructures of the solidified AZ91 alloys usually consist of (Mg) grains and mainly the Al12Mg17 phase at the grain boundaries [2006Wan; 2009Kab]. Similar microstructures are observed in other AZ series alloys [2011Wu].
The following suggestions are useful to help you analyze the simulated results and interpret experimental observations.
When working in Thermo‑Calc with Scheil simulations, you use either the Scheil Calculator (in Graphical Mode) or the Scheil module (in Console Mode). The fundamental calculation engine is the same but you access the settings in different ways.
Check the Calculated Phase Fractions
It is recommended to always check the calculated phase fractions in your results before comparing the simulation with experimentally observed microstructures.
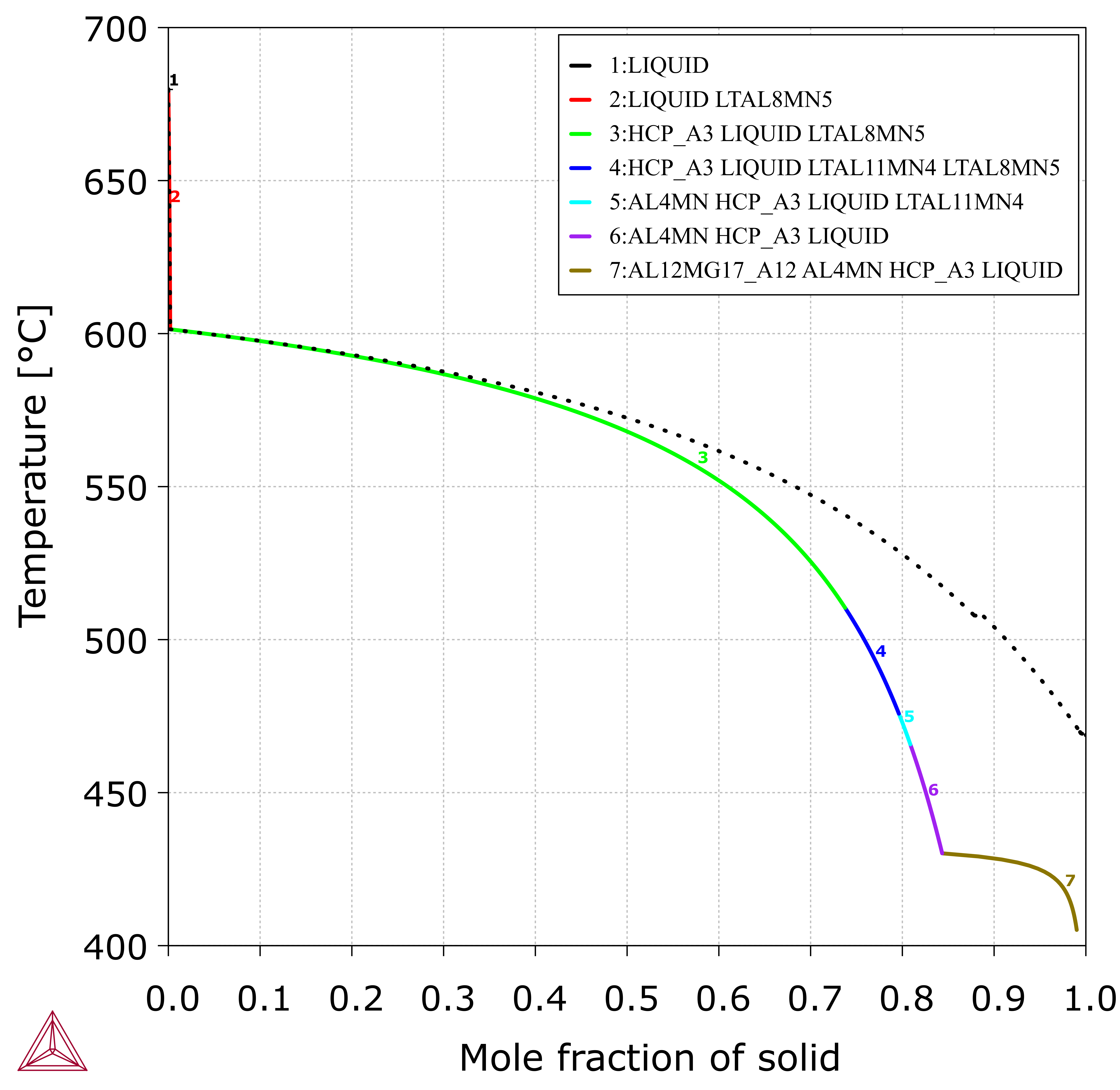
The following example uses an alloy composition of 8.82 wt. % Al, 0.91 wt. % Zn, 0.31 wt. % Mn, Mg balance. Figure 1 plots the total fraction of solid phases against temperature from the solidification calculation of an AZ91 alloy.
The calculation predicts the formation of three Al-Mn compounds, Al8Mn5, Al11Mn4, and Al4Mn, in addition to the experimentally observed phases, HCP_A3-(Mg) and Al12Mg17. At first glance, the prediction of phase formation is obviously different from the experimental observations.
Figure 1: Scheil solidification with all the phases included and the total fraction of solid phases against temperature. The solid line corresponds to the Scheil calculation and the dashed line to the equilibrium calculation.
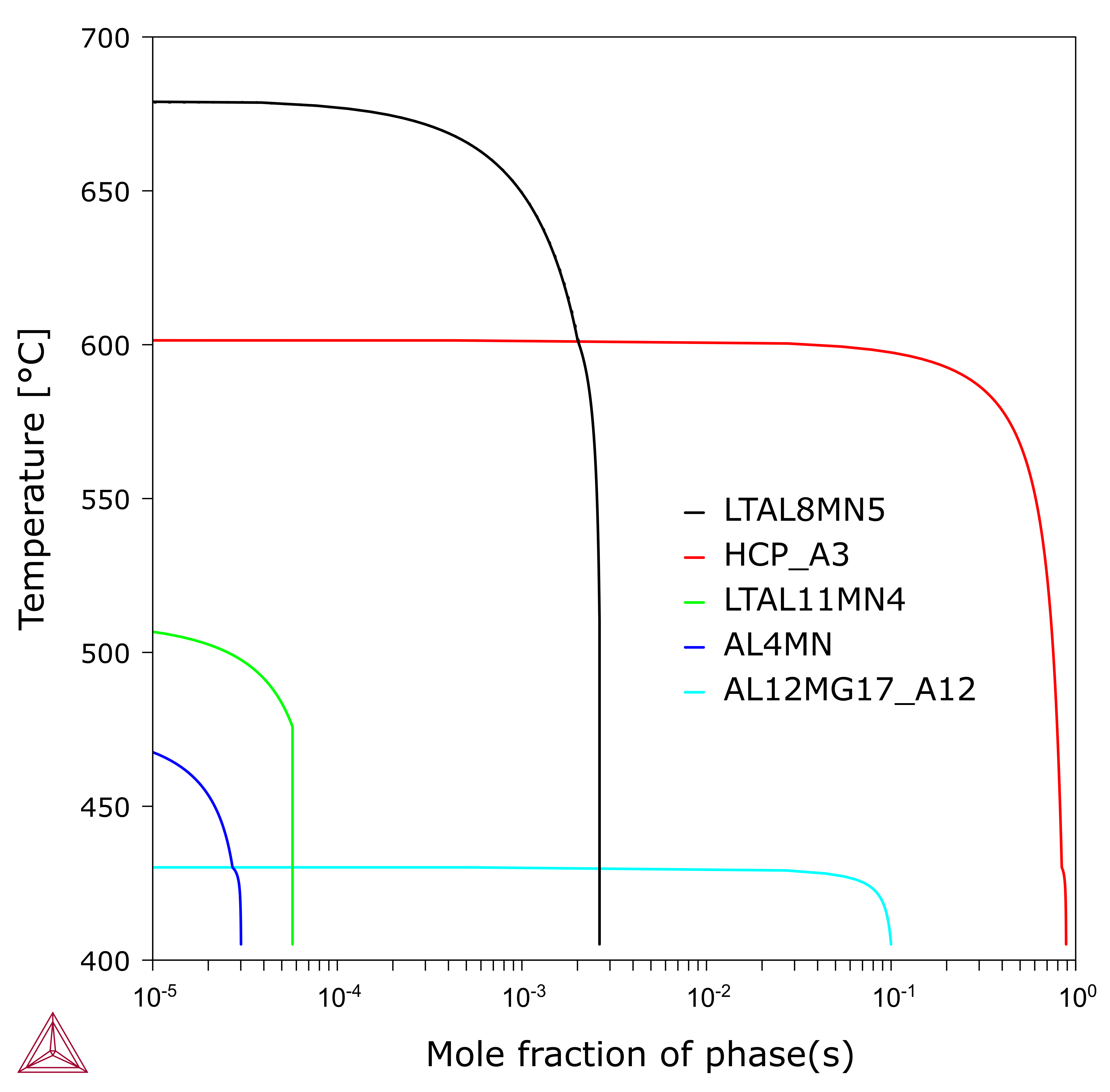
However, when you compare this to Figure 2, the amounts of the Al-Mn compounds are negligible. Of these, Al8Mn5 has the highest amount but less than 0.3% and the other two are less than 0.01%.
Figure 2: Scheil solidification with all the phases included and the Al-Mn compounds are in negligible amounts.
The negligible amounts may be easily overlooked in experimental examination due to the small value. Especially, the Al8Mn5 phase forms in prior to (Al) and it would probably not appear at grain boundaries.
You can always choose to neglect the discrepancies or plan to utilize more advanced techniques in further experimental investigations if the identification of these phases is important.
Add Mn as an Alloying Element
Mn is usually added as an alloying element in Mg-Al based alloys (including AZ, AM, and AS series). This element is believed to be able to increase resistance against corrosion and prevent soldering in high-pressure die casting of magnesium alloys. Furthermore, Al8Mn5 usually forms at the beginning of the solidification of the AZ series Mg alloys and it had been reported that this phase might act as potent nucleation sites for (Mg). Although this ability of Al8Mn5 was doubted by the work of Wang et al. [2010Wan], this phase does precipitate in the solidified microstructures, which confirms the present simulation. It is not easy to experimentally identify the manganese aluminides in Al-Mg based alloys, especially Al11Mn4 [2004Bar] and Al4Mn [2004Dhu].
You can observe that using the TCS Mg-based Alloys Database (TCMG) the Scheil simulation agrees with experimental observations. Further, the theoretical calculations can predict the phases that have a minor or trace amount, which are otherwise difficult to experimentally identify.
Reject Phases with Negligible Amounts
Practically, during the simulation you can reject the phases that have negligible amounts.
It can be distracting to include insignificant phases in diagrams. Practically, you can do a second-round simulation excluding those phases. This is accomplished by first rejecting all phases and then restoring those of interest.
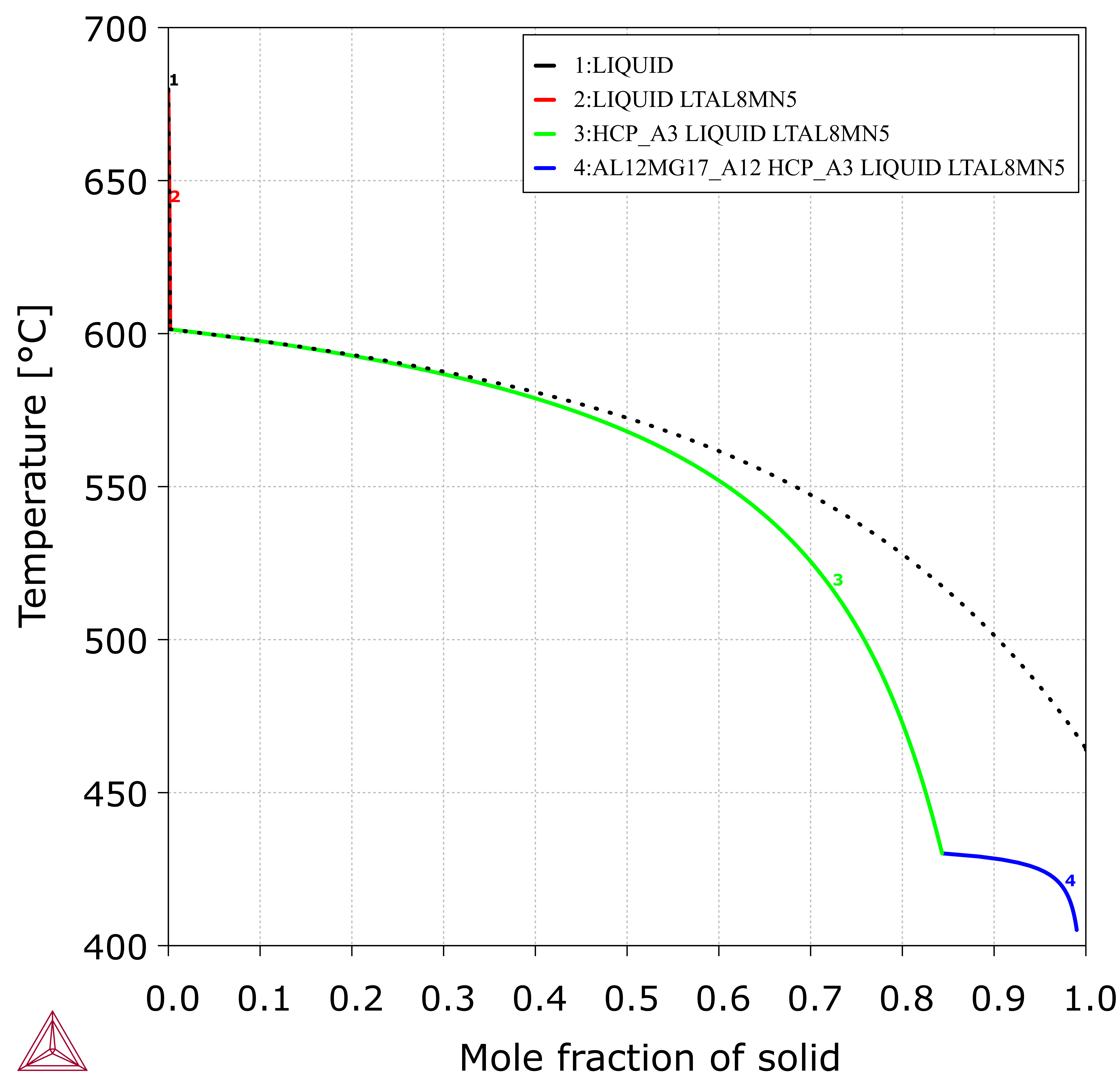
The following example uses the same alloy composition as in the previous examples, 8.82 wt. % Al, 0.91 wt. % Zn, 0.31 wt. % Mn, Mg balance. Figure 3 shows the simulated Scheil solidification curve with only liquid, Al8Mn5, HCP_A3 and Al12Mg17 included. The solidification curve is almost the same as that in Figure 1 as is the phase formation of the major phases. However, with those minor phases excluded it is much easier to analyze the simulated results.
Figure 3: Scheil solidification of the AZ91 alloy with only Liquid, Al8Mn5, Hcp_A3, and Al12Mg17 included.
Adjust the Minor Alloying Elements
In a simulation, you can exclude or include some of the minor alloying elements that have no significant effects on the solidification sequence.
Instead of rejecting the minor phases during the set up of your solidification simulation, you can also exclude some of the minor alloying elements that have no significant effects on the solidification sequence. This is particularly useful when the alloy contains a large number of alloying elements and when many intermetallic phases may potentially form. In the case of the AZ91 alloy, you can consider excluding Mn in the simulation. This has the advantage of getting rid of all the Mn-bearing compounds including Al8Mn5.
However, whether to include or exclude a minor alloying element depends on the purpose of the simulation. For example, sometimes it is important to know the impact of impurities on solidification and formation of the primary phase.
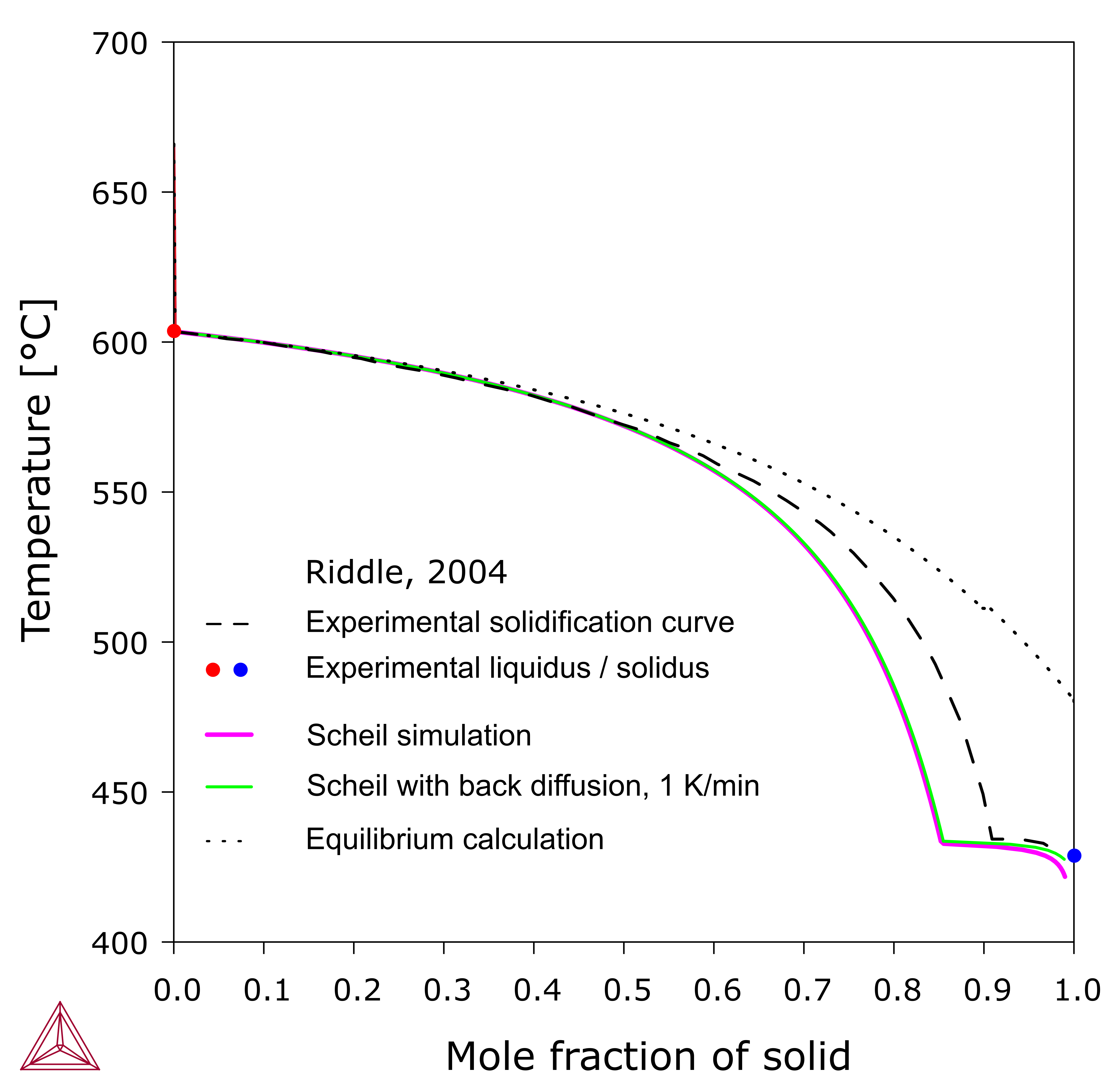
Figure 4 shows a Scheil simulation of an AZ91D alloy by considering a small amount (about 0.15 wt.%) of Fe impurity. The solidification starts with the formation of B2-AlFe, followed by Al8Mn5. It was experimentally observed by [2018Zen] that Al8Mn5 actually nucleated on the B2 phase. In this case, the impurity, Fe, as well as the minor alloying element, Mn, has to be considered in order to account for the experimental observations.
Interpret the Thermal Analysis Data
It is useful to spend time interpreting the thermal analysis data.
Al8Mn5 is the primary phase during the solidification of the AZ91 alloy. The liquidus temperature (i.e. the start of the solidification of Al8Mn5) is calculated to be 680 °C.
The first arrested thermal effect in some thermal analysis experiments, e.g., 600.6 °C [2014Bak] or 604 °C [2004Rid], does not correspond to the liquidus temperature, but is related to the start of the solidification of (Mg), which is 601.4 °C according to the Scheil calculation.
Since the amount of Al8Mn5 is low, the heat effect corresponding to the solidification of Al8Mn5 is too small to be readily detected. Precisely conducted thermal analysis, however, can still detect the thermal effect. Thorvaldsen and Aliravci [1992Tho] successfully measured the Al8Mn5 liquidus in several AZ91 alloys with 9.1 wt. % Al, 1.53 wt. % Zn and Mn varying from 0 to 1 wt.%. It has been shown in (in calculation examples) that these data can be well accounted for by the phase diagram calculated using the TCS Mg-based Alloys Database (TCMG).
Experimental Techniques and Back Diffusion
It is recommended to make moderate use of Scheil solidification since a real solidification is expected to be between a Scheil solidification simulation and an equilibrium solidification simulation.
The Scheil solidification simulation provides a good approximation to non-equilibrium solidification processes and is widely used. However, it should be noted that any experimental technique has disadvantages and blind spots. Since transition temperatures are often measured with thermal analysis, the heat effect must be significant enough to be detected. Although it is well-known, as predicted here, the primary phase in AZ91 alloys is Al8Mn5, but its amount is too small to be detected. The liquidus temperature was measured to be 603.5 °C [2004Rid], in comparison with 680 °C as predicted in the Scheil simulation in Figure 4. However, the seemingly different results actually imply a good agreement. The experimental temperature actually corresponds to the start precipitation of (Mg) grains, i.e. HCP_A3, where the thermal effect became noticeable. The agreement is very good. Therefore, one has to understand how the results were measured and what could make a difference, while comparing the results with the simulations.
Figure 4 also shows the solidification curve derived from thermal analysis. A good agreement was seen between the experimental results and the Scheil simulation in the early stage. When the solid fraction is larger than 50%, however, there is a noticeable or significant difference. Such experimental results should be considered qualitative, since quite a few assumptions are made during the derivation, for example, that (1) the heat is proportional to the solid phase fraction and (2) the heat transfer is immediate. Many factors may add to the uncertainties.
It should be pointed out that, conventional Scheil simulation also has its own limitation, which is the assumption that the diffusion in solid phases is negligible. Although this is reasonable in many cases, back diffusion in solid phases might sometimes become noticeable either in systems where diffusion is relatively fast or during slow cooling. In Thermo‑Calc you can take the impact of back diffusion into account, as shown in Figure 4, which includes the results from a Scheil simulation with back diffusion. It results in a good agreement with the experimental solidus temperature, as well as the late solidification stage [2004Rid], although the intermediate range almost remains the same. As above, the experimental solidification curve can only be considered qualitative.
Figure 4: Comparison of the simulated equilibrium and Scheil solidification profiles with experimental thermal analysis [2004Rid].
The alloy composition used in this example is 8.7 wt. % Al, 0.5 wt. % Zn, 0.26 wt. % Mn, Mg balance.
As expected, the experimental profile is located between the two solidification simulations, while it can be better approximated with the Scheil simulation. The start temperature of the solidification of (Mg) and the eutectic temperature of L = (Mg) + Al12Mg17, together with the profile shape, are all accounted for by the Scheil simulation. However, the solid fractions from the Scheil simulation and the experiment do not agree well with each other in the region between 60% and 90%. This deviation indicates that the diffusion in the solid phases is not negligible (as assumed in Scheil) even though it is truly slow.
A real solidification process could much more approach to either of the two solidification simulations and deviate from the other, depending on the experimental conditions and the alloy systems. In order to account for the differences between the simulation and the experimental observation, you should consider the effect of back diffusion in a Scheil simulation.
References
[1992Tho] A. Thorvaldsen, C. A. Aliravci, Adv. Prod. Fabr. Light Met. Met. Matrix Comp., in Proceedings of the International Symposium, p. 277 (1992).
[2004Bar] L. P. Barber, Characterization of the solidification behavior and resultant microstructures of Magnesium-Aluminum alloys, Master’s thesis, Worcester Polytechnic Institute, Worcester, MA (2004).
[2004Rid] Y. W. Riddle, M. M. Makhlouf, “Characterizing solidification by non-equilibrium thermal analysis” in Magnesium Technology 2003 (TMS (The Minerals, Metals & Materials Society), 2004), pp. 101–106.
[2004Dhu] E. Dhuka, N. Lohja, H. Oettel, D. Heger, Precipitation in Mg alloy AZ61 in dependence of various heat treatments processes. Metalurgija. 10, 233–241 (2004).
[2006Wan] Y. Wang, G. Liu, Z. Fan, Microstructural evolution of rheo-diecast AZ91D magnesium alloy during heat treatment. Acta Mater. 54, 689–699 (2006).
[2009Kab] F. Kabirian, R. Mahmudi, Effects of Zirconium Additions on the Microstructure of As-Cast and Aged AZ91 Magnesium Alloy. Adv. Eng. Mater. 11, 189–193 (2009).
[2010Wan] Y. Wang, M. Xia, Z. Fan, X. Zhou, G. E. Thompson, The effect of Al8Mn5 intermetallic particles on grain size of as-cast Mg–Al–Zn AZ91D alloy. Intermetallics. 18, 1683–1689 (2010).
[2011Wu] L. Wu, F. Pan, M. Yang, J. Wu, T. Liu, As-cast microstructure and Sr-containing phases of AZ31 magnesium alloys with high Sr contents. Trans. Nonferrous Met. Soc. China. 21, 784–789 (2011).
[2014Bak] D. P. Bakke, D. H. Westengen, “The Role of Rare Earth Elements in Structure and Property Control of Magnesium Die Casting Alloys” in Essential Readings in Magnesium Technology (Wiley, Hoboken, NJ, USA, 2014), pp. 313–318.
[2018Zen] G. Zeng, J. W. Xian, and C. M. Gourlay, Nucleation and growth crystallography of Al8Mn5 on B2-Al(Mn,Fe) in AZ91 magnesium alloys, Acta Mater., vol. 153, pp. 364–376 (2018).