Mg-Y-Zn(-Zr) Alloys
Rare earth elements are major alloying elements or important additives in a wide range of Mg alloys. They help to improve high-temperature strength and creep resistance. The investigation of Mg-RE-TM alloys has attracted extra interest due to the finding of the long-period stacking-ordered (LPSO) phases.
RE = rare earth element, such as Gd, Tb, Dy, Ho, Er, Tm, or Y and TM = mostly transition metals such as Co, Ni, Cu, and Zn or the post-transition metal Al.
Also see another example about Long-period Stacking-ordered (LPSO) Structures.
The (0001) basal slip dominates the plastic deformation of the LPSO phase and the deformation kinks form when the basal slip is suppressed. The plastic anisotropy of the LPSO phase significantly contributes to the strengthening of (Mg)/LPSO two-phase alloys by enhancing grain refinement of the (Mg) grains. Among the Mg-RE-TM alloy systems, Mg-Y-Zn has been most extensively investigated and it forms the two most common types of LPSO structures, 14H and 18R.
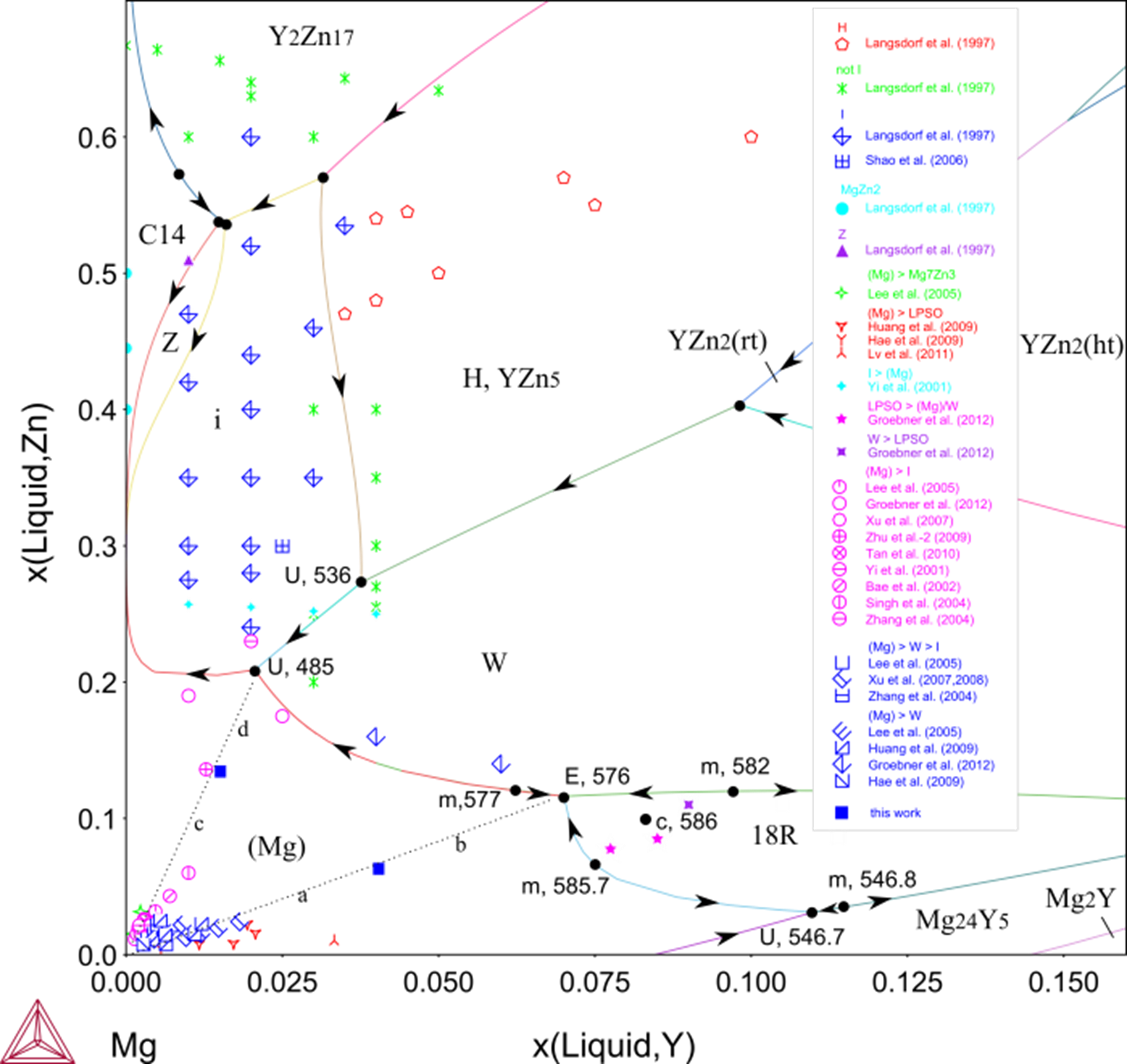
The TCS Mg-based Alloys Database (TCMG) is used in this example to examine these Mg-Y-Zn(-Zr) alloys. Figure 1 shows the calculated Mg-Y-Zn liquidus projection in the Mg-rich region, which is validated with experimental results from as-cast Mg-Y-Zn(-Zr) alloys.
A small amount of Zr does not change the formation of major phases.
Phase formation sequences are marked for the most Mg-rich alloys in the range of < 10 at.% Y and < 25 at.% Zn. Not only the primary crystallization regions but also the compositions of the invariant equilibria agree with experimental data. As you can see, two arbitrary dividing lines “ab” and “cd” can be drawn based on the phase formation sequences and they extend towards “E 576” and “U 485”, respectively.
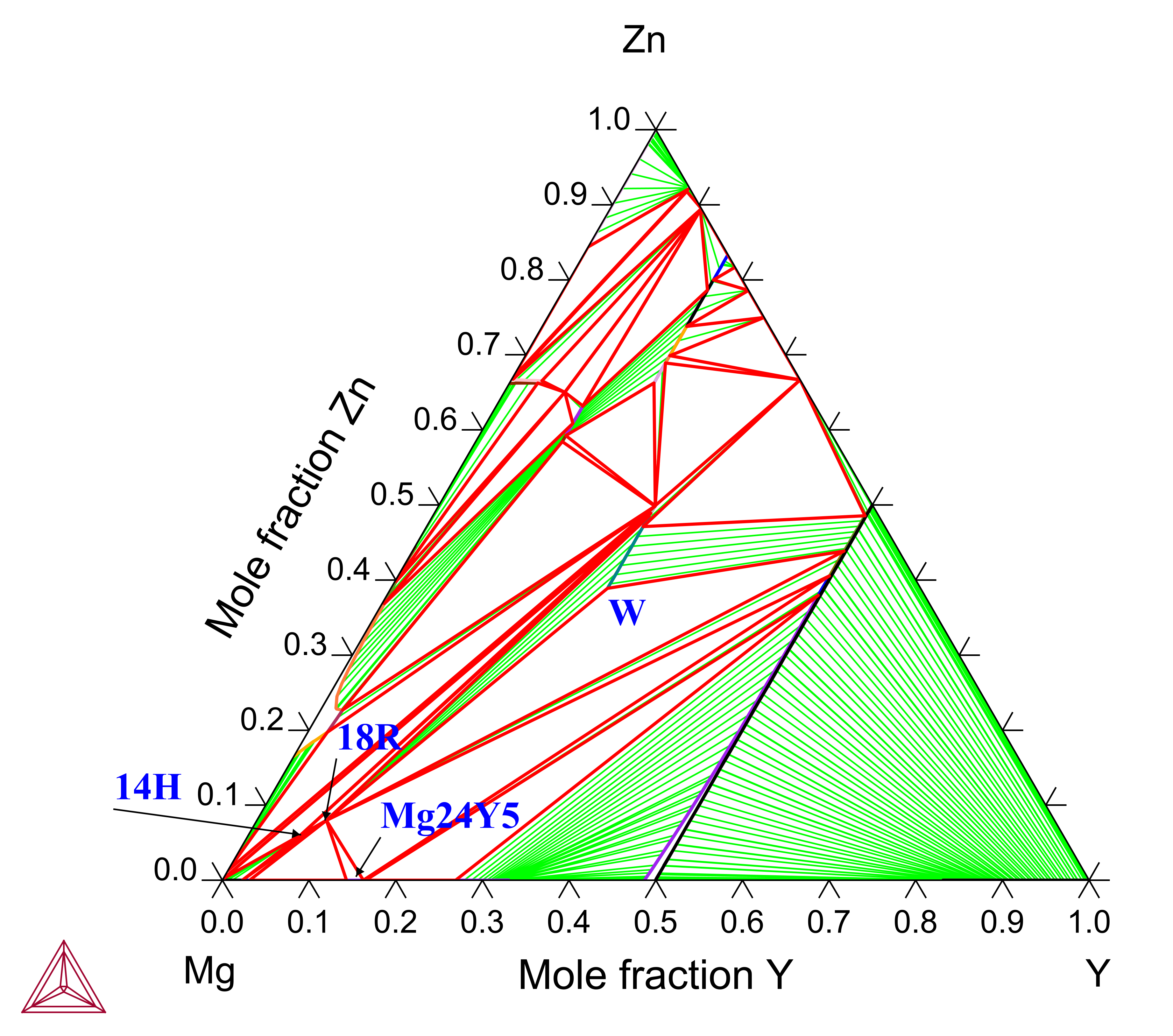
For the rest of the alloy compositions, at which only primary solidifying phases are known, the results can also be well accounted for. The 14H phase can only form via solid phase transformations. Its existence can be seen in the isothermal section at 773 K (Figure 2).
Figure 1: Calculated Mg-Y-Zn liquidus projection in the Mg-rich corner and the Mg-Zn vicinity [2015Che].
Figure 2: Calculated Mg-Y-Zn isothermal section at 773 K, showing the presence of 14H (LPSO), 18R (LPSO), W (Mg3R), I, Z, and H (YZn5) [2015Che].
Reference
[2015Che] H. Chen, Thermodynamic assessment of the Gd-Mg-Zn and Mg-Y-Zn systems in TCMG4, Thermo‑Calc Software (2015).