Impurity Elements
The impurity level in titanium alloys inevitably accounts for varying features such as β-transus temperature and phase fractions vs. temperature.
It is known that low amounts of interstitial impurities (such as O, C, and N) affect phase equilibria and transformations in both Ti- and TiAl‑based alloys. Attributing to the high susceptibility to contamination of titanium alloys, it is suggested to rationally consider the effects from impurities for simulations.
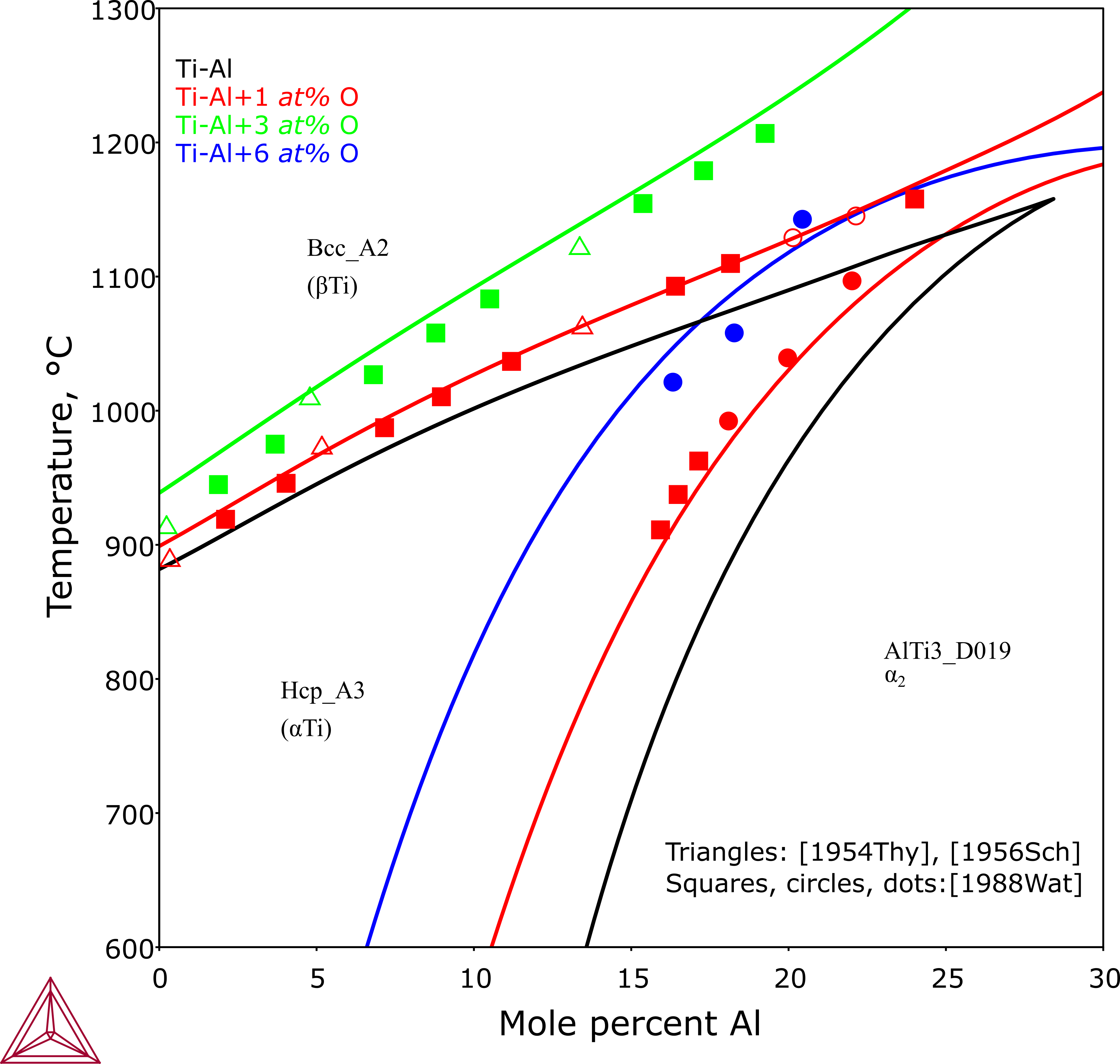
This plot shows with increasing amount of oxygen, both Hcp_A3 and AlTi3_D019 phases get stabilized.
Hydrogen is also incorporated in the thermodynamic TCS Ti/TiAl-based Alloys Database (TCTI), where hydrogen solubilities in solutions and stabilities of metal hydrides have been assessed.
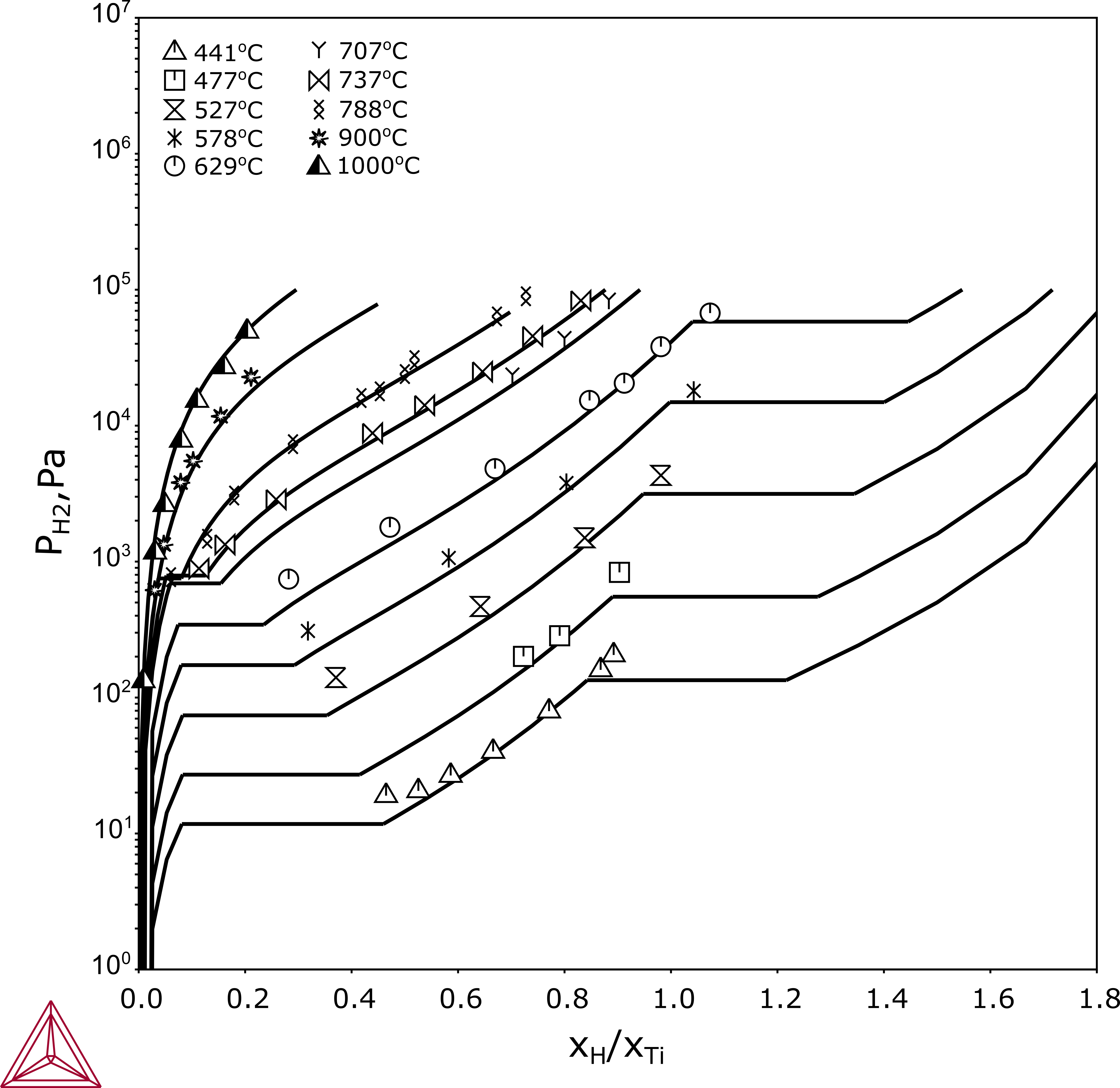
Figure 2: Calculated pressure-composition isotherms of the Ti-H system compared with experimental data cited in [1987San].
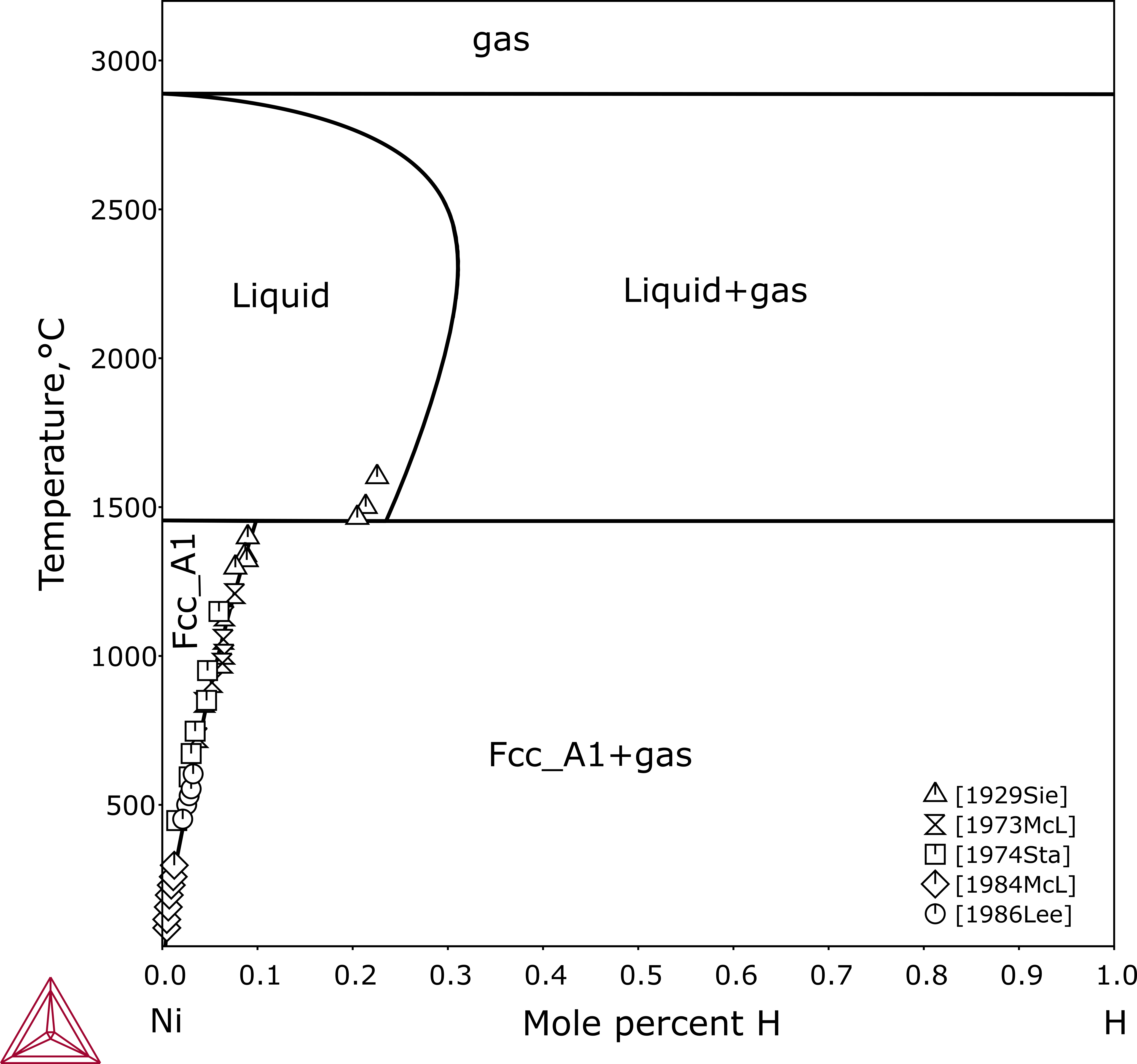
Figure 3: Calculated Ni-H phase diagram with experimental data from [1929Sie; 1973McL; 1974Sta; 1984McL; 1986Lee].
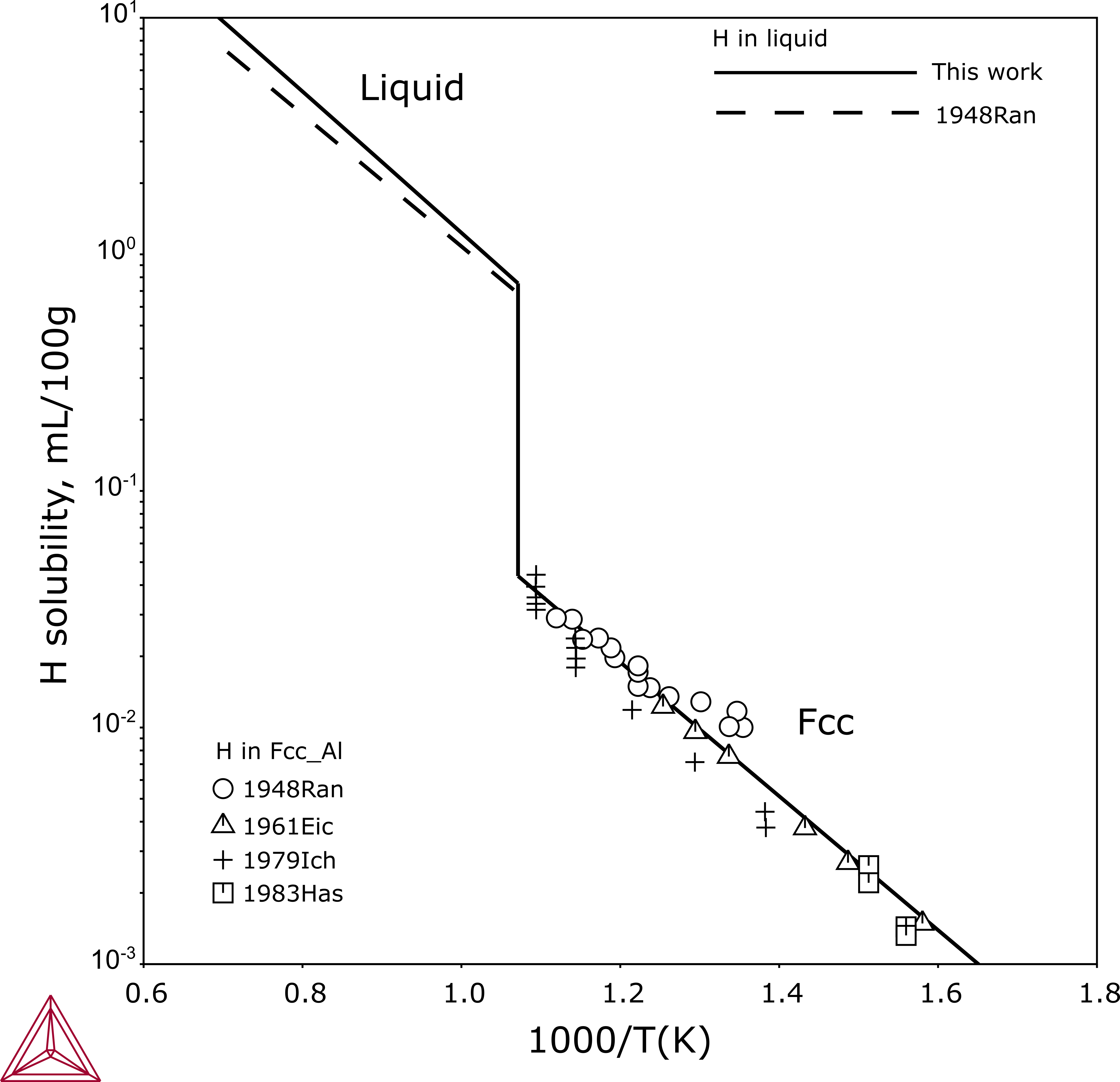
Figure 4: Calculated solubility of H in Al compared with experimental information from [1948Ran; 1961Eic; 1979Ich; 1983Has].
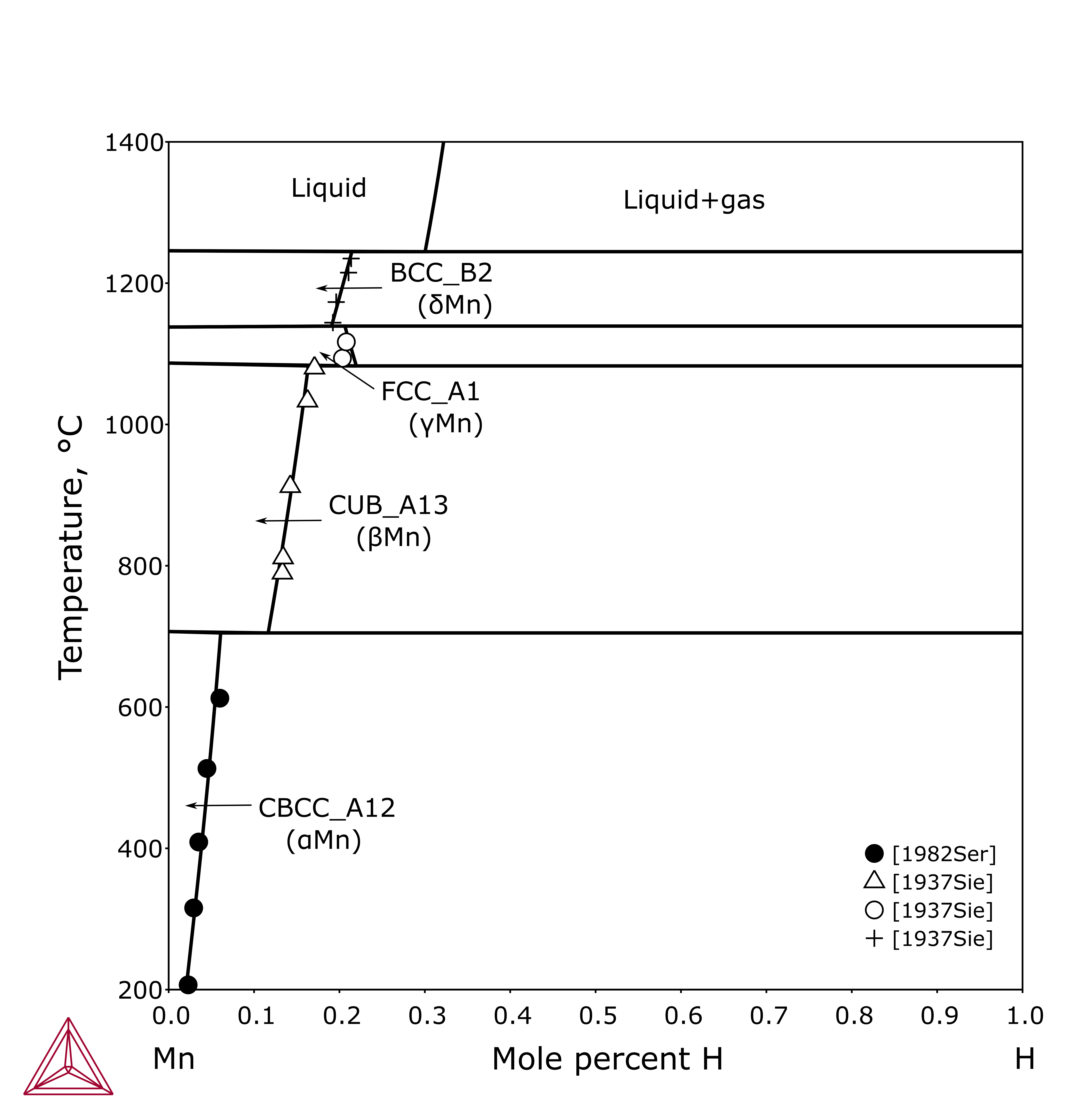
Figure 5: Calculated solubility of H in solid Mn compared with experimental data [1937Sie; 1982Ser].
References
[1929Sie] A. Sieverts, Die Aufnahme von Gasen durch Metalle (Absorption of Gases by the Metals). Int. J. Mater. Res. (Zeitschrift für Met.) 21, 37–46 (1929).
[1937Sie] A. Sieverts, H. Moritz, Manganese and Hydrogen. Z Phys. Chem., Abt. A. 180, 249–263 (1937).
[1948Ran] C. E. Ransley, D. E. Neufeld, The solubility of hydrogen in liquid and solid aluminum, J. Inst. Met., 74, 599-604 (1948).
[1954Thy] R. J. Van Thyne, H. D. Kessler, Influence of Oxygen, Nitrogen, and Carbon on the phase relationships of the Ti-Al system. Trans. AIME. 6, 193–199 (1954).
[1956Sch] T. H. Schofield, A. E. Bacon, The Constitution of the Titanium-rich Alloys of Titanium, Aluminium and Oxygen. J. Inst. Met. 85, 193–195 (1956).
[1961Eic] W. Eichenauer, K. Hattenbach, A. Pebler, Die Löslichkeit von Wasserstoff in festem und flüssigem Aluminium (The solubility of hydrogen in solid and liquid aluminum). Int. J. Mater. Res. (Zeitschrift für Met.) 52, 682–684 (1961).
[1973McL] R. B. McLellan, W. A. Oates, The solubility of hydrogen in rhodium, ruthenium, iridium and nickel, Acta Metall., 21, 181–185 (1973).
[1974Sta] S. W. Stafford, R. B. McLellan, The solubility of hydrogen in nickel and cobalt, Acta Metall., 22, 1463– 1468 (1974).
[1979Ich] M. Ichimura, M. Imabayashi, M. Hayakawa, Measurement of the Diffusion Coefficient and Solubility of Hydrogen in Solid Aluminum. J. Japan Inst. Met. 43, 876–883 (1979).
[1982Ser] H. P. Serdyuk, B. I. Shapovalov, The constitution diagram of the Manganese-Hydride system. lzv. EU.Z, Chernaya Met. 8, 70–74 (1982).
[1983Has] E. Hashimoto, T. Kino, Hydrogen diffusion in aluminium at high temperatures. J. Phys. F Met. Phys. 13, 1157–1165 (1983).
[1984McL] R. B. McLellan, P. L. Sutter, Thermodynamics of the hydrogen-nickel system, Acta Metall., 32, 2233–2239 (1984).
[1986Lee] S.-M. Lee, J.-Y. Lee, The trapping and transport phenomena of hydrogen in nickel, Metall. Trans. A 17, 181–187 (1986).
[1987San] A. San-Martin, F. D. Manchester, The H−Ti (Hydrogen-Titanium) system. Bull. Alloy Phase Diagrams. 8, 30–42 (1987).
[1988Wat] R. M. Waterstrat, “Effect of Interstitial Elements on Phase Relationships in the Titanium-Aluminum System (NISTIR 88-3856)” (U.S. Dept. of Commerce, Arlington, Virginia, 1988).