Nitrogen Alloyed Duplex Stainless Steels (DSS)
HT Phase Equilibria
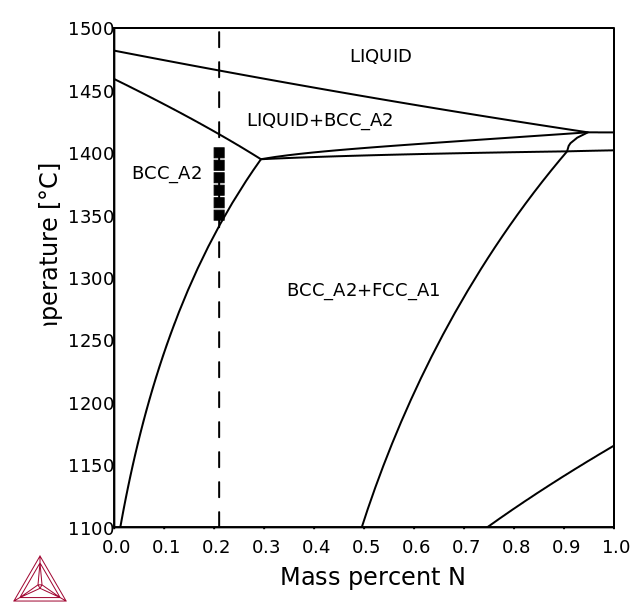
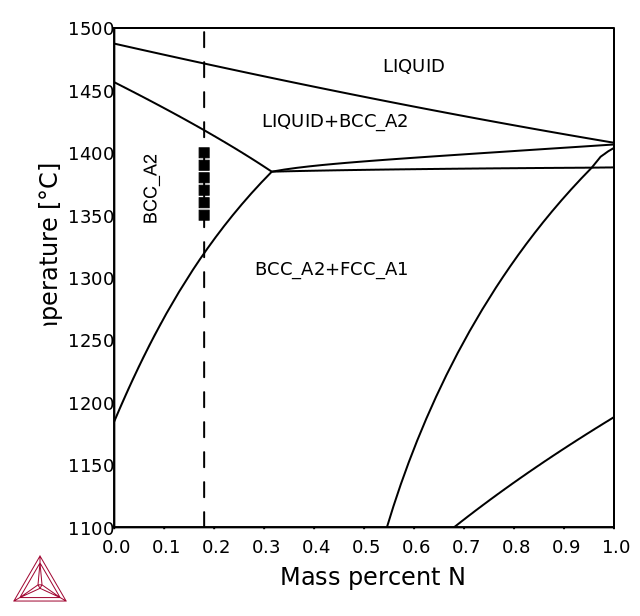
The duplex steels are designed to solidify in the single-phase ferritic mode with formation of austenite by precipitation in the solid state [1992Nil; 2017Pet].
The high-temperature phase equilibria calculations with TCS Steel and Fe-alloys Database (TCFE) show the interval below solidus where the material is fully ferritic.
|
|
Figure 1: Calculated isopleth sections for LDX2101 grade (left) and 2205 grade (right) with nitrogen contents up to 1 wt. %. The nitrogen content of the alloy is indicated by the dashed lines. Experimental data is from the FROST project – Internal report.
Limits of Nitrogen Solubility
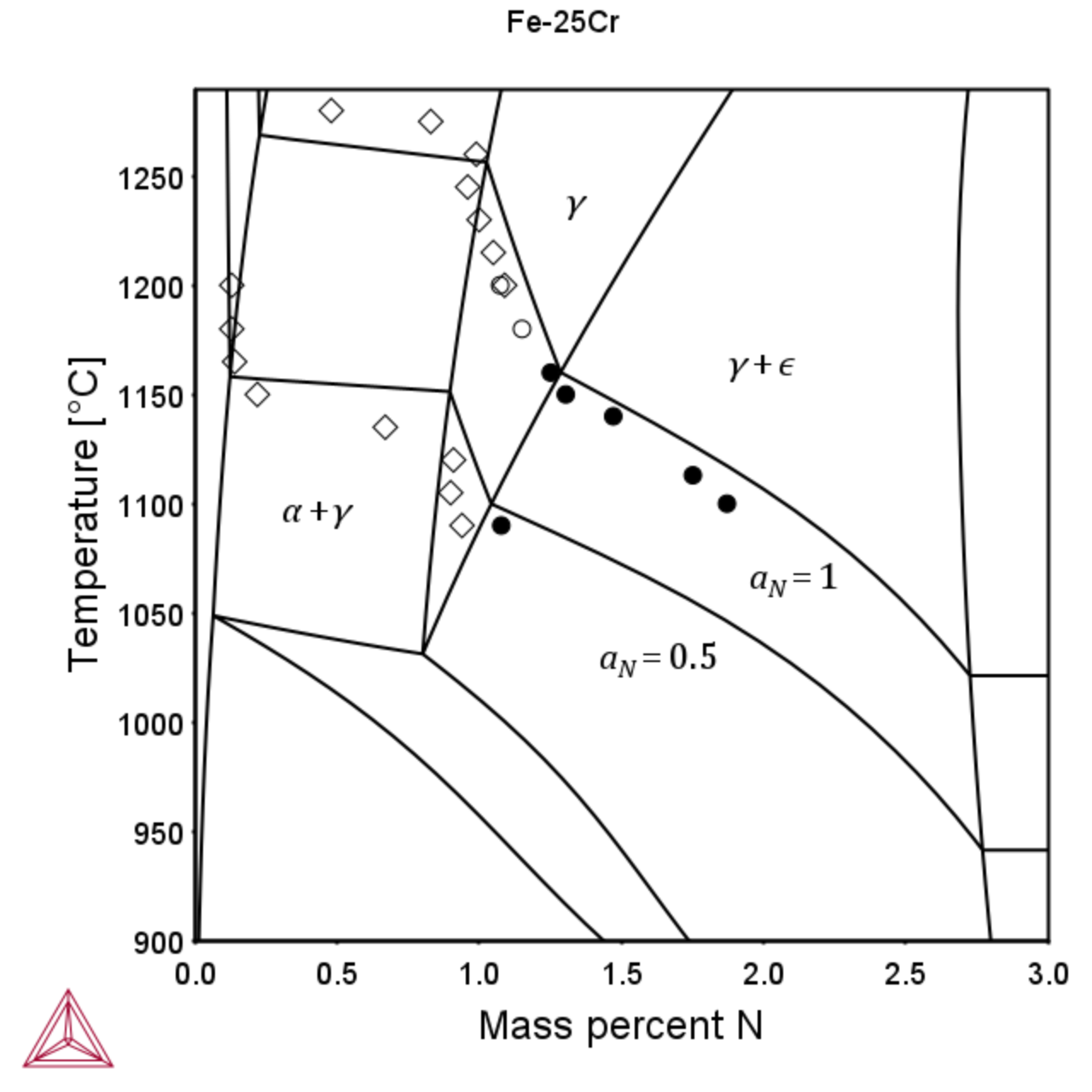
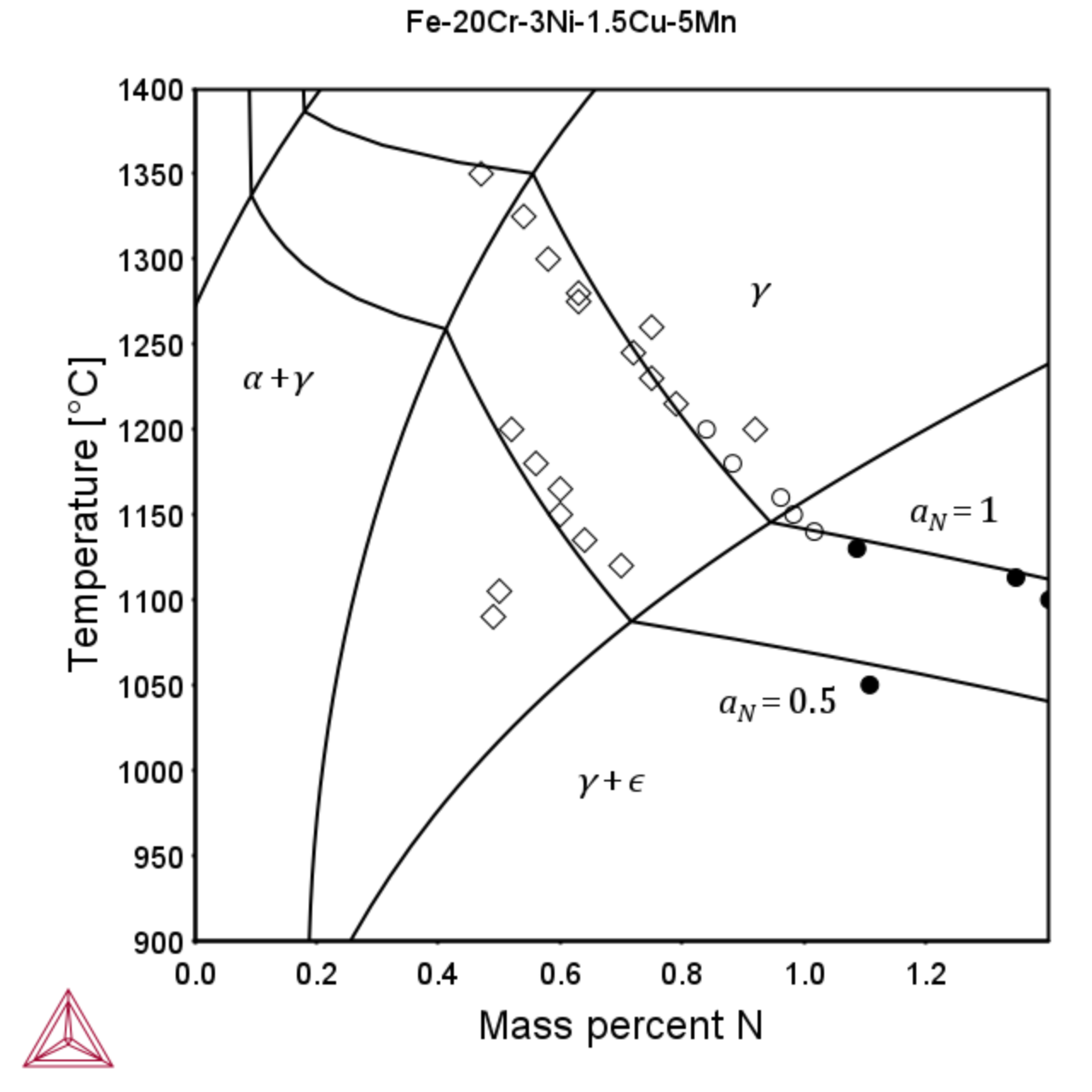
The TCS Steel and Fe-alloys Database (TCFE) can also be used to calculate the limits of nitrogen solubility in austenite and ferrite at various nitrogen activities as shown below. Experimental data is from the FROST project – Internal report.
|
|
Figure 2: Isopleth for the Fe-25Cr (left) and Fe-20Cr-5Mn-3Ni-1.5Cu-N (right) system showing the phase regions of main interest and the isoactivity line aN = 1 and the iso-activity line for aN=0.5.
η-silicide Precipitation
The η-silicide (η-nitride) has a diamond cubic structure with typical composition Cr3Ni2SiN and is indicated to be the equilibrium nitrogen bearing phase in austenitic stainless steels alloyed with nitrogen according to research made by Jargelius-Pettersson [1998Jar]. Experimental information found in the literature together with new experimental information within internal projects were used to assess the thermodynamic description of the η- silicide (ETA_M5SIN) in TCS Steel and Fe-alloys Database (TCFE). In Table 1 the composition of some studied alloys are shown and in Table 2 a comparison is made between observed and calculated composition of η-silicide.
Table 1. Composition (wt. %) of two of the alloys studied by Jargelius-Pettersson [1998Jar].
|
Alloy |
C |
Si |
Mn |
P |
S |
Cr |
Ni |
Mo |
N |
|---|---|---|---|---|---|---|---|---|---|
|
L3 |
0.014 |
0.54 |
1.44 |
0.009 |
0.003 |
19.8 |
25.0 |
4.59 |
0.21 |
|
B3 |
0.014 |
0.56 |
5.24 |
0.012 |
0.004 |
20.2 |
18.5 |
4.28 |
0.44 |
Table 2. Comparison between measured and calculated composition of η-silicide in alloys B3 and L3 at 800 °C [1998Jar].
|
Alloy |
at. % |
Cr |
Ni |
Mo |
Fe |
Si |
N |
|---|---|---|---|---|---|---|---|
|
B3 |
Exp. |
30 |
25.5 |
12 |
5 |
13 |
14.5 |
|
Calc. |
28.4 |
25.9 |
14.5 |
2.7 |
14.3 |
14.3 |
|
|
L3 |
Exp |
24 25.1 |
25.0 24.7 |
15.0 15.2 |
4.5 8.0 |
13 (14.3) |
18.5 (14.3) |
|
Calc. |
29.5 |
26.4 |
13.3 |
2.2 |
14.3 |
14.3 |
References
[1992Nil] J.-O. Nilsson, Super duplex stainless steels, Mater. Sci. Technol., 8 (8), 685–700 (1992).
[1998Jar] R.F.A. Jargelius-Pettersson, Precipitation trends in highly alloyed austenitic stainless steels, Zeitschrift Für Met., 89, 177–183 (1998).
[2017Pet] N. Pettersson, S. Wessman, S. Hertzman, and A. Studer, High-temperature phase equilibria of duplex stainless steels assessed with a novel in-situ neutron scattering approach, Metall. Mater. Trans. A, 48 (4), 1562–1571 (2017).