FCC+BCC High Entropy Alloys (HEAs)
These examples show some classical calculations of the equilibrium phase stabilities for the FCC+BCC high entropy (HEA) alloys when using the TCS High Entropy Alloys Database (TCHEA).
FCC Medium (MEA) and High Entropy (HEA) Alloys
BCC Medium (MEA) and High Entropy (HEA) Alloys
HCP Medium (MEA) and High Entropy (HEA) Alloys
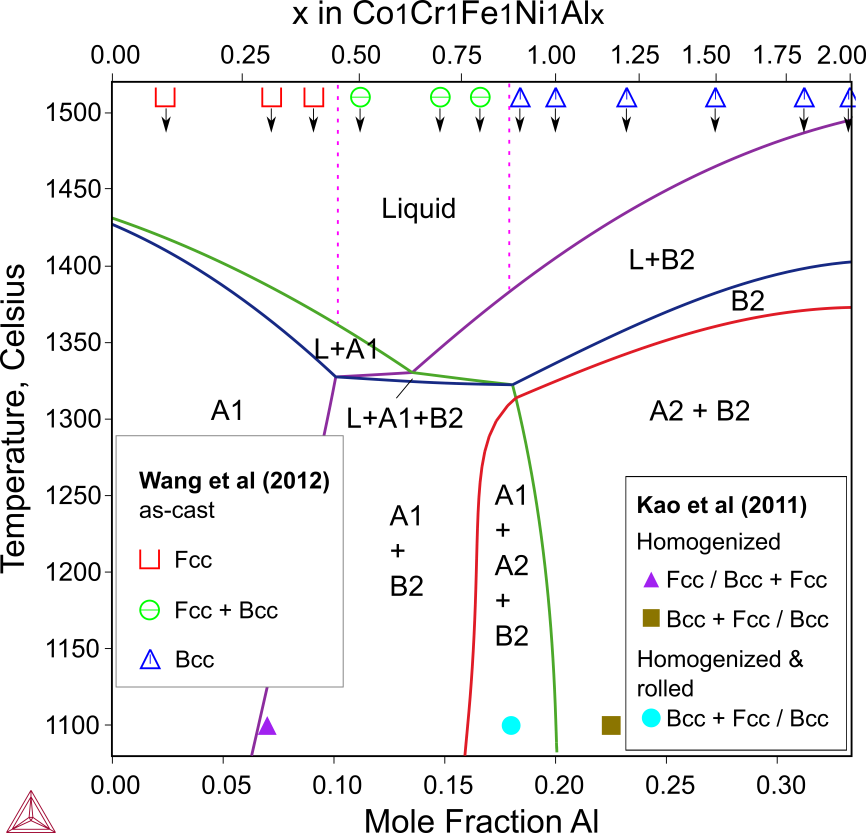
AlxCoCrFeNi FCC+BCC HEAs
This example shows the effect of the Al addition on the phase formation in the equiatomic CoCrFeNi base medium entropy alloy (MEA). The phase formed in as-cast base alloy is dominated by FCC_A1. With a small amount of Al addition (<10 at.% Al), only FCC forms in the as-cast alloys. Adding about 11.11 % Al (x = 0.5) results in the formation of a second phase the B2 phase. The region roughly between 10 and 18 % Al is near the eutectic reaction (eutectic composition 13.98 % Al or x = 0.65), where both FCC and BCC form in as-cast alloys. Further increasing the Al content causes the exchange of the solidification sequence and the primary formation of B2. When the Al content increased up to more than 18.37 at.% Al (x = 0.9), FCC_A1 might be absent during the fast solidification, thus only BCC forms.
The phase formation also depends on the actual experimental conditions, especially the cooling rate and the heat treatment. The data from Wang [2012Wan] indicate the compositions of the investigated as-cast alloys and the observed phases. The data from Kao et al. [2011Kao] are the composition limits for forming BCC, FCC and BCC+FCC in as-cast alloys or alloys homogenized at 1000 °C.
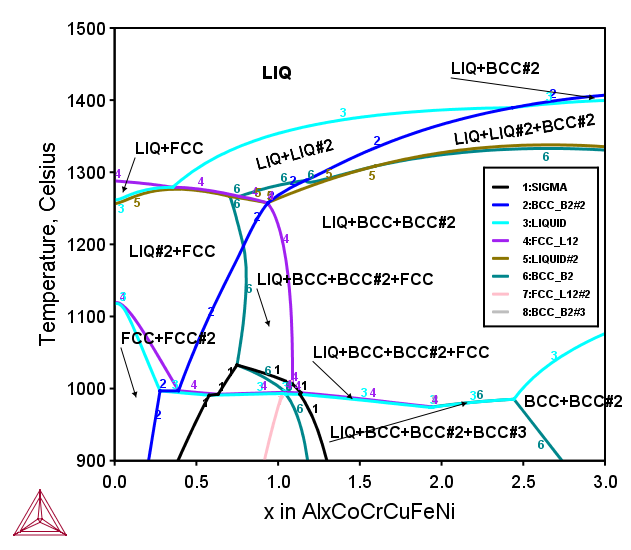
AlxCoCrCuFeNi FCC+BCC HEAs
Figure 2: Calculated phase equilibria in the isopleth for the series of the AlxCoCrCuFeNi senary alloys, where x=0-3 represents moles of atoms of the element Al [2017Mao].
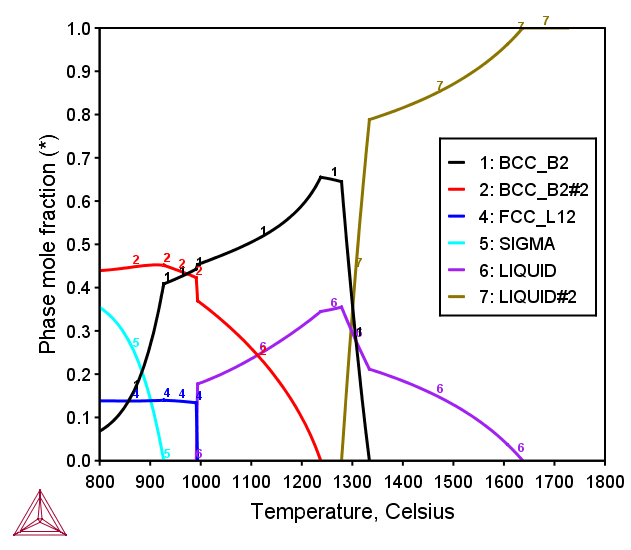
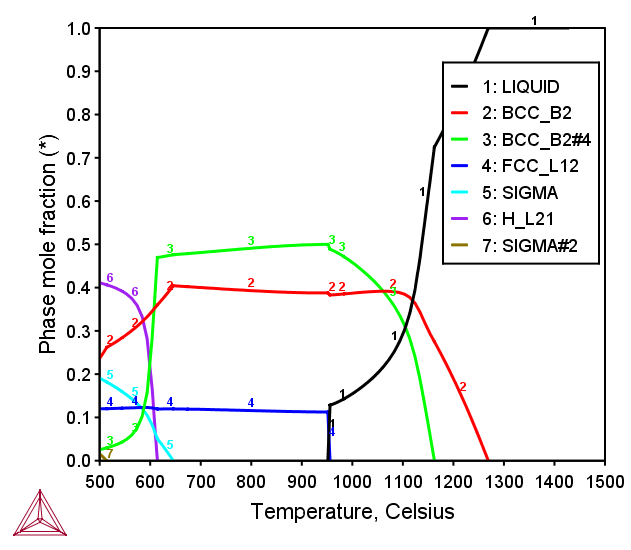
AlCoCrCuFeNiV FCC+BCC HEA
Figure 3: Calculated mole fraction of equilibrium phases at various temperatures in the AlCoCrCuFeNiV septenary alloy with equiatomic ratio [2017Mao].
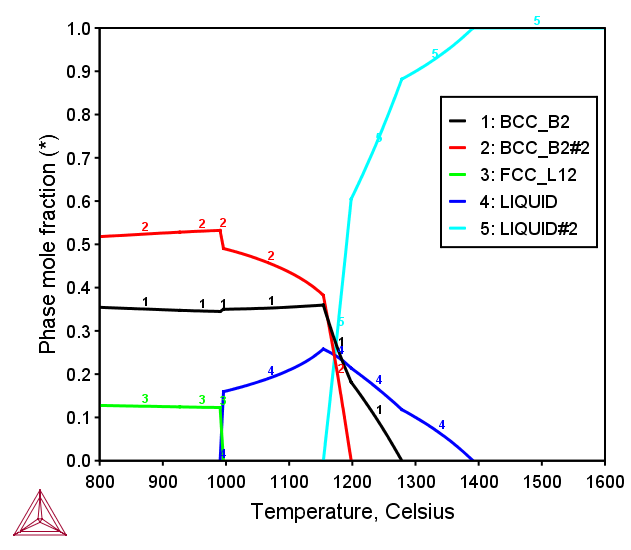
AlCoCrCuFeNiTiV FCC+BCC HEA
Figure 4: Calculated mole fraction of equilibrium phases at various temperatures in the AlCoCrCuFeNiTiV octonary alloy with equiatomic ratio [2017Mao].
AlCoCrCuFeMnNiTiV FCC+BCC HEA
Figure 5: Calculated mole fraction of equilibrium phases at various temperatures in the AlCoCrCuFeMnNiTiV ennead alloy with equiatomic ratio [2017Mao].
References
[2011Kao] Y.-F. Kao, S.-K. Chen, T.-J. Chen, P.-C. Chu, J.-W. Yeh, S.-J. Lin, Electrical, magnetic, and Hall properties of AlxCoCrFeNi high-entropy alloys. J. Alloys Compd. 509, 1607–1614 (2011).
[2012Wan] W.-R. Wang, W.-L. Wang, S.-C. Wang, Y.-C. Tsai, C.-H. Lai, J.-W. Yeh, Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics. 26, 44–51 (2012).
[2017Mao] H. Mao, H.-L. Chen, Q. Chen, TCHEA1: A Thermodynamic Database Not Limited for “High Entropy” Alloys. J. Phase Equilibria Diffus. 38, 353–368 (2017).
[2018Che] H.-L. Chen, H. Mao, Q. Chen, Database development and Calphad calculations for high entropy alloys: Challenges, strategies, and tips. Mater. Chem. Phys. 210, 279–290 (2018).