FCC Medium (MEA) and High Entropy (HEA) Alloys
These examples show some classical calculations of the equilibrium phase stabilities for the FCC medium entropy (MEA) and high entropy (HEA) alloys when using the TCS High Entropy Alloys Database (TCHEA).
BCC Medium (MEA) and High Entropy (HEA) Alloys
HCP Medium (MEA) and High Entropy (HEA) Alloys
FCC+BCC High Entropy Alloys (HEAs)
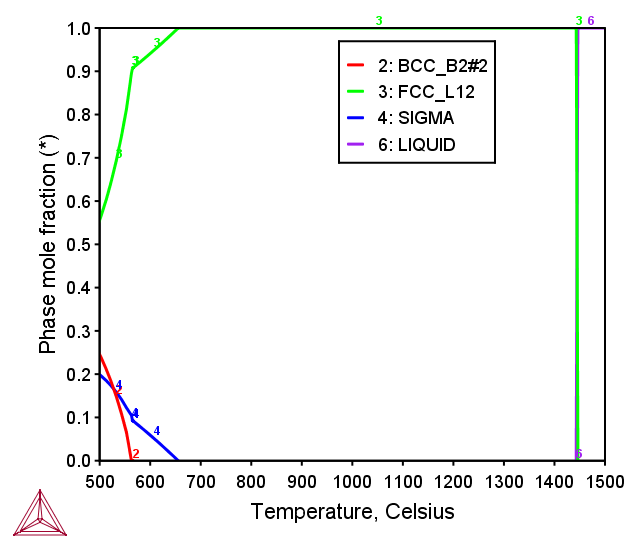
CoCrFeNi FCC MEA
Figure 1: Mole fraction of equilibrium phases at various temperatures in the CoCrFeNi equiatomic alloy [2017Mao].
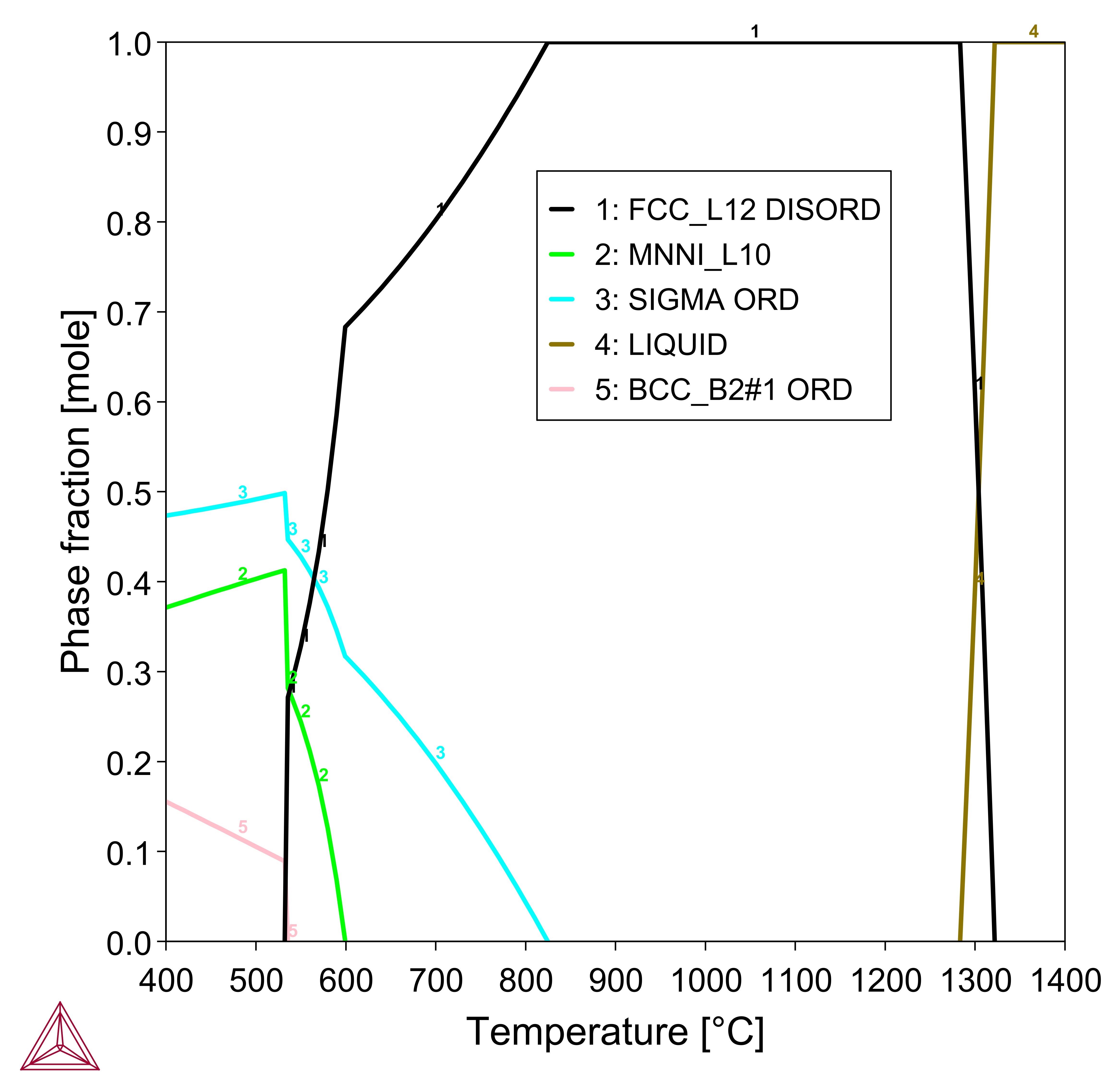
CoCrFeMnNi FCC HEA
Figure 2: Mole fraction of equilibrium phases at various temperatures in the CoCrFeMnNi equiatomic alloy, a.k.a. the Cantor alloy [2004Can] .
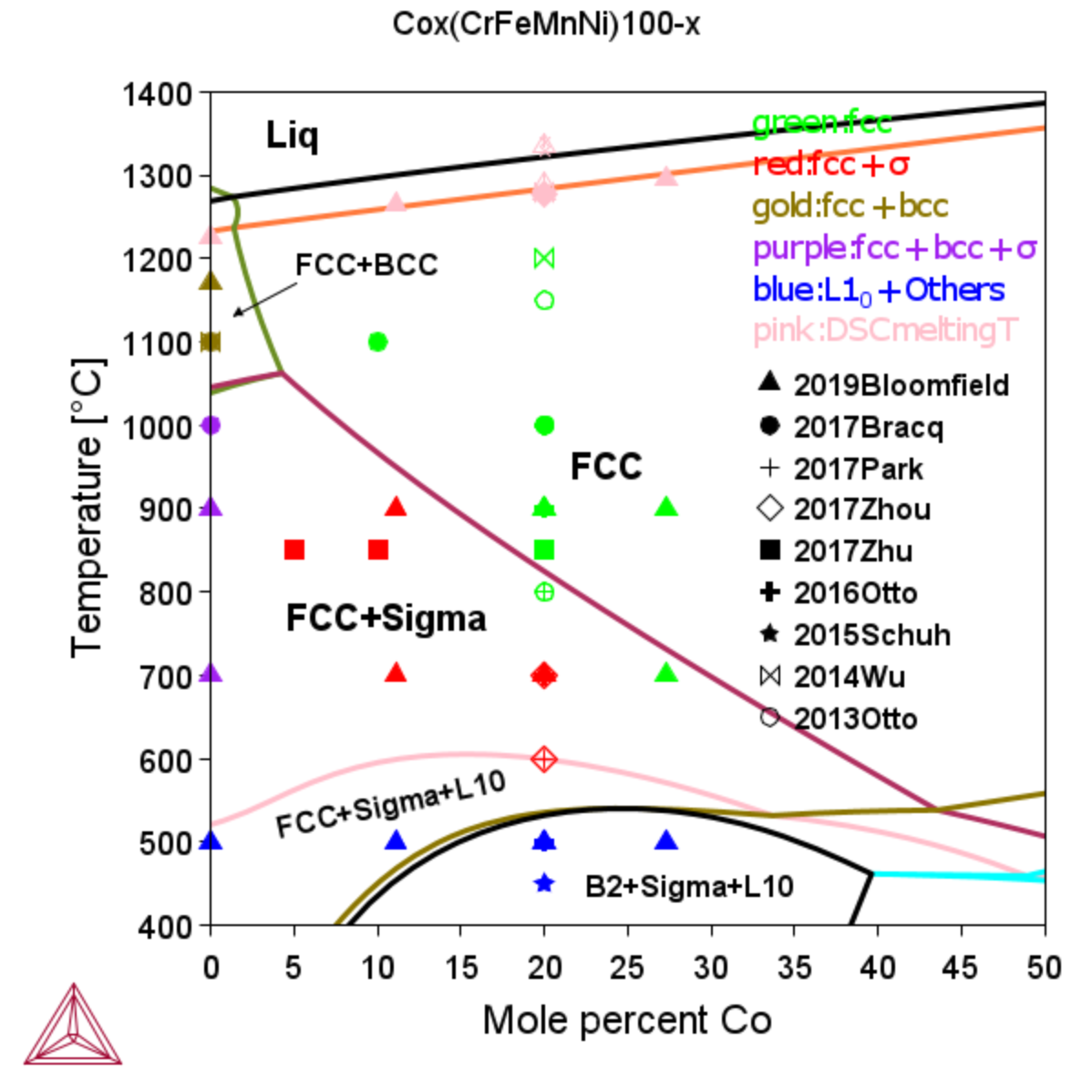
Cox(CrFeMnNi)100-x FCC HEA
TCS High Entropy Alloys Database (TCHEA) predicts well the melting properties (solidus and liquidus), and stability range of FCC, BCC, sigma, and L10 phases.
Figure 3: Calculated phase equilibria in the isopleth of Cox(CrFeMnNi)100-x, where x represents at% of Co. In the plot, symbols represent experimentally observed phases. These are coded in color for different phase assemblages, e.g. green for single FCC, and red for FCC+Sigma two-phase coexists. Different symbols represent different experimental work. Filled and empty symbols stand for long (>=24 h) and short (<24 h) annealing, respectively.
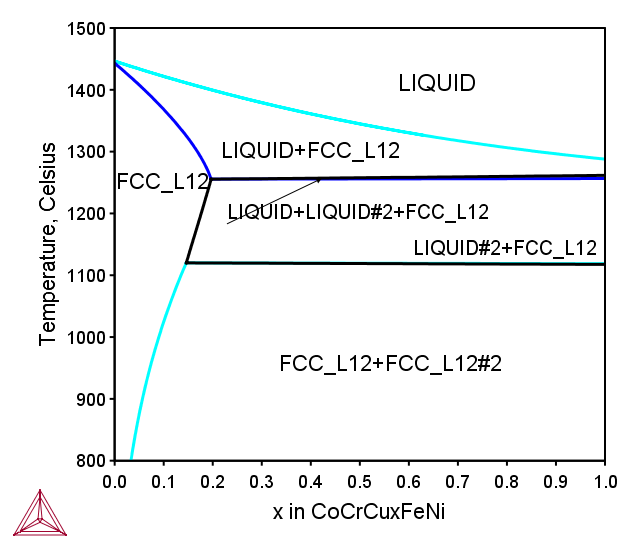
CoCrCuxFeNi FCC HEA
Figure 4: Calculated phase equilibria in the isopleth for the series of the CoCrCuxFeNi quinary alloys, where x=0-1 represents moles of Cu atoms [2017Mao].
References
[2004Can] B. Cantor, I. T. H. Chang, P. Knight, A. J. B. Vincent, Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A. 375–377, 213–218 (2004).
[2017Mao] H. Mao, H.-L. Chen, Q. Chen, TCHEA1: A Thermodynamic Database Not Limited for “High Entropy” Alloys. J. Phase Equilibria Diffus. 38, 353–368 (2017).