Solidification Simulation (Equilibrium vs Scheil)
Equilibrium and Scheil simulations can be used to study the solidification microstructure evolution. Calculated fractions and compositions of solid and liquid phases help understand the segregation profile and guide homogenization temperatures/time.
Read more about Scheil Solidification Simulations on our website, including how to select the right model for your simulation. If you are in Thermo‑Calc, press F1 to search the help to learn about using Scheil.
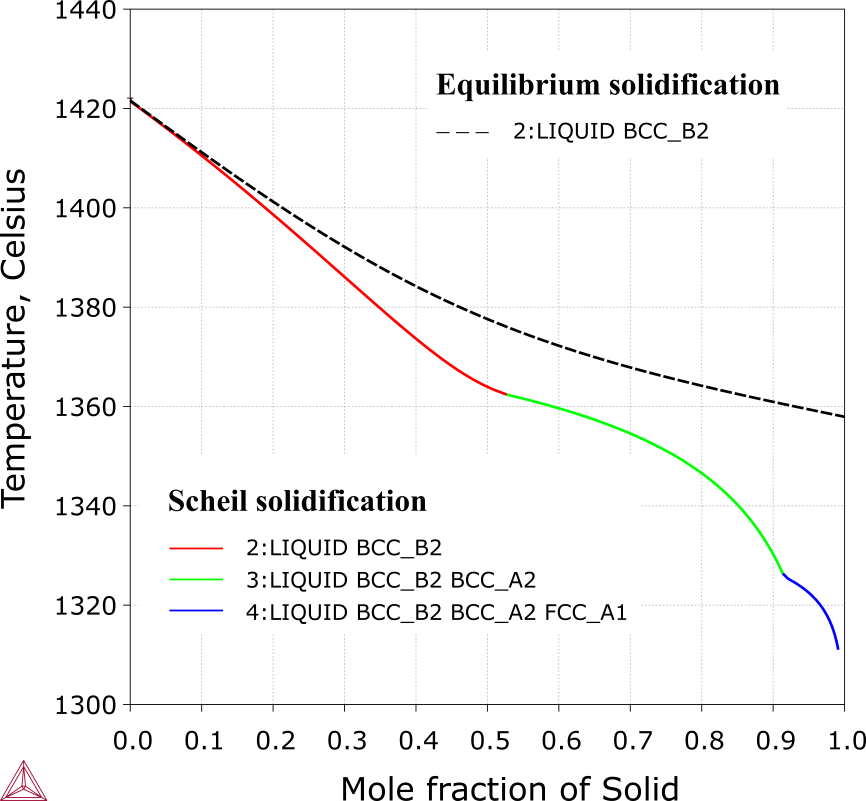
CoCrFeNiAl1.1 Alloy
Figure 1: Equilibrium (in dashed line) and Scheil (in solid line) solidification simulations of the CoCrFeNiAl1.1 alloy (21.57 at.% Al) [2018Che].
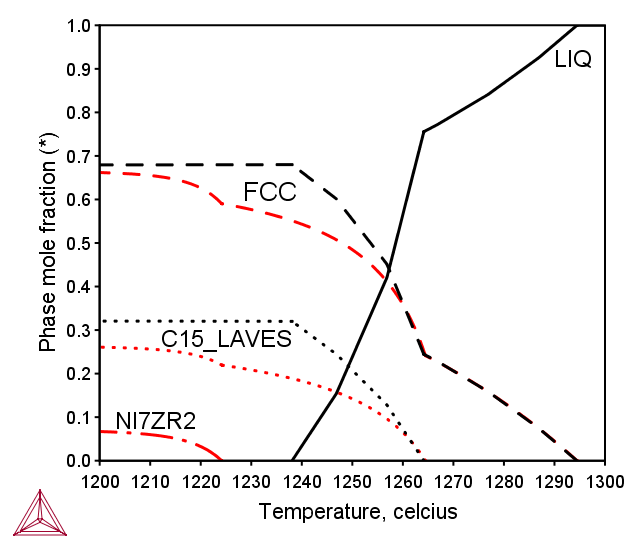
CoCrFeNiZr0.4 Alloy
Scheil simulations are helpful to understand the solidification behavior during fast cooling. In the as-cast CoCrFeNiZr0.4 alloy, in addition to the FCC and C15_Laves phase, a minor amount of Ni7Zr2 phase was observed [2017She]. According to the equilibrium calculation, and shown by the black curves in Figure 2, no Ni7Zr2 phase was predicted. However, the Scheil simulation, the red curves in the same figure, does predicts a minor fraction of solidification of Ni7Zr2. You can consider that the global equilibrium calculation and the Scheil simulation mimic two extreme conditions for the solidification process. A real case should happen at the condition in between, depending on the kinetic conditions.
Starting with Thermo‑Calc version 2020a, it is also possible to take the back-diffusion into account using the Scheil Calculator in Graphical Mode.
Figure 2: Predicted solid phase mole fractions by equilibrium (black curves) and Scheil simulation (red curves) of the CoCrFeNiZr0.4 alloy [2017Mao].
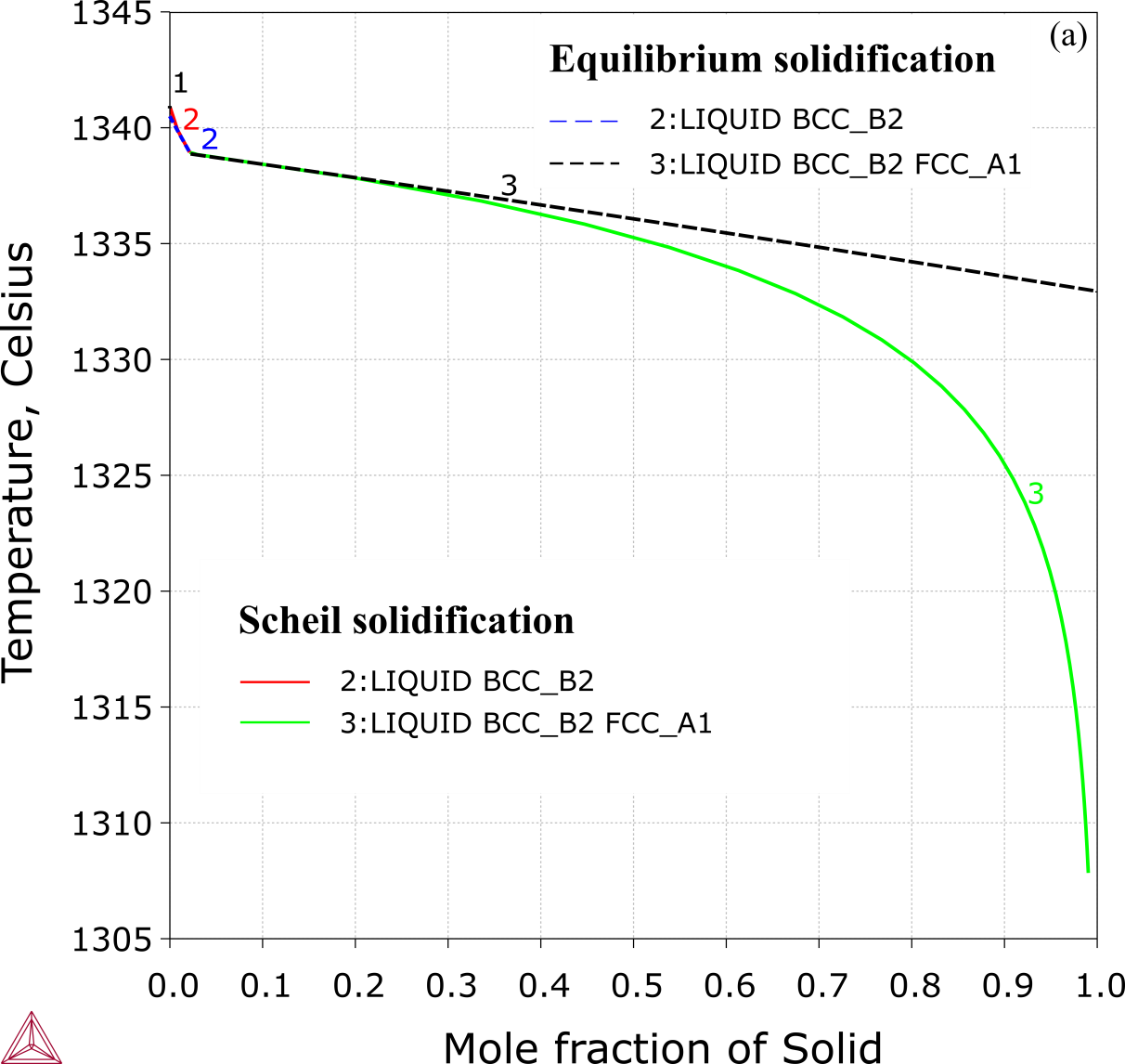
CoCrFeNiAl0.65 Alloy
Figure 3: Solidification simulation of the eutectic alloy CoCrFeNiAl0.65 (13.98 at.% Al): Phase formation sequence and solid phase fractions from equilibrium (in dashed line) and Scheil simulation (solid line) [2018Che].
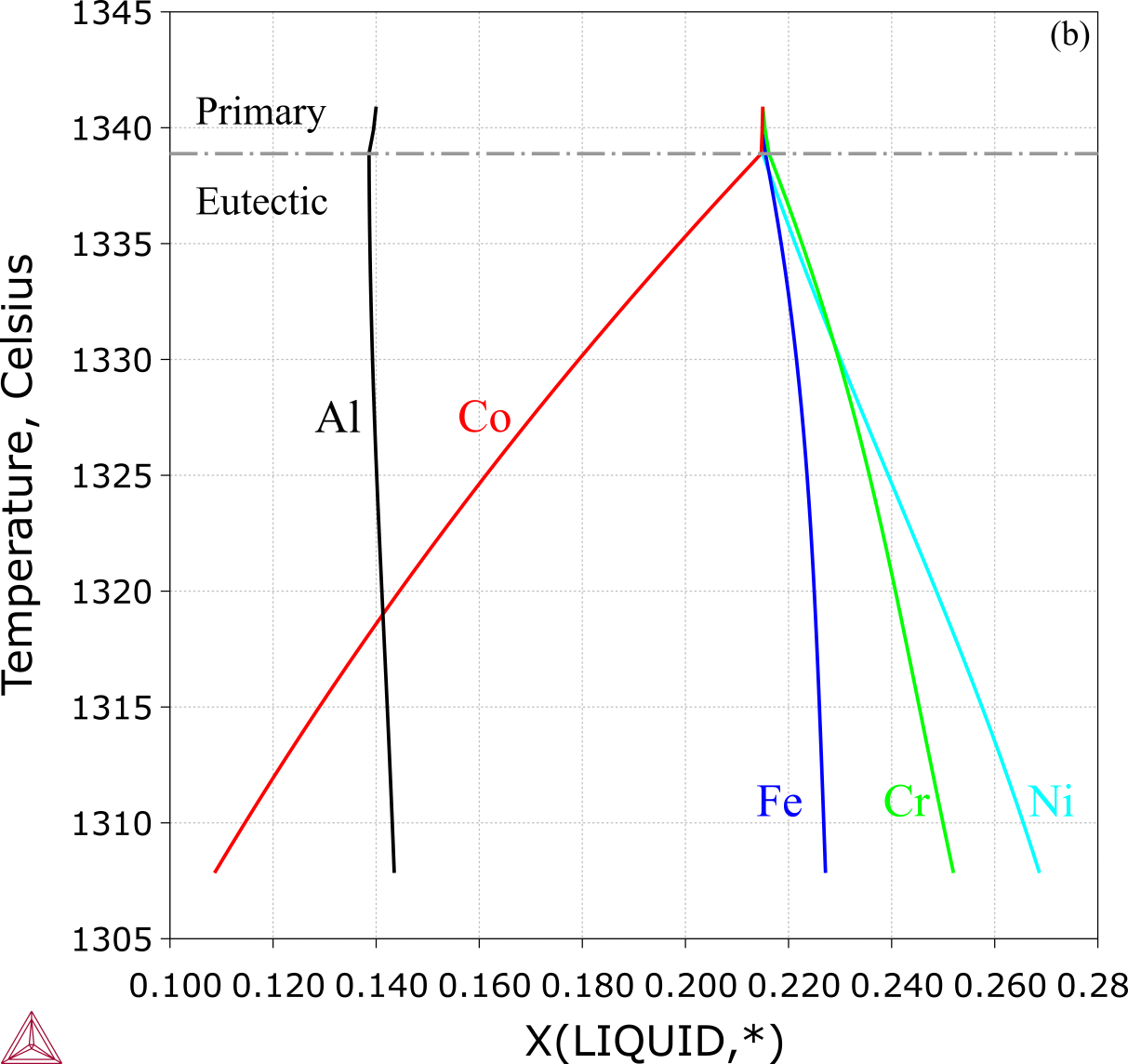
Figure 4: Solidification simulation of the eutectic alloy CoCrFeNiAl0.65 (13.98 at.% Al): Liquid phase composition from a Scheil simulation [2018Che].
CoCrCuFeMnNi Alloy
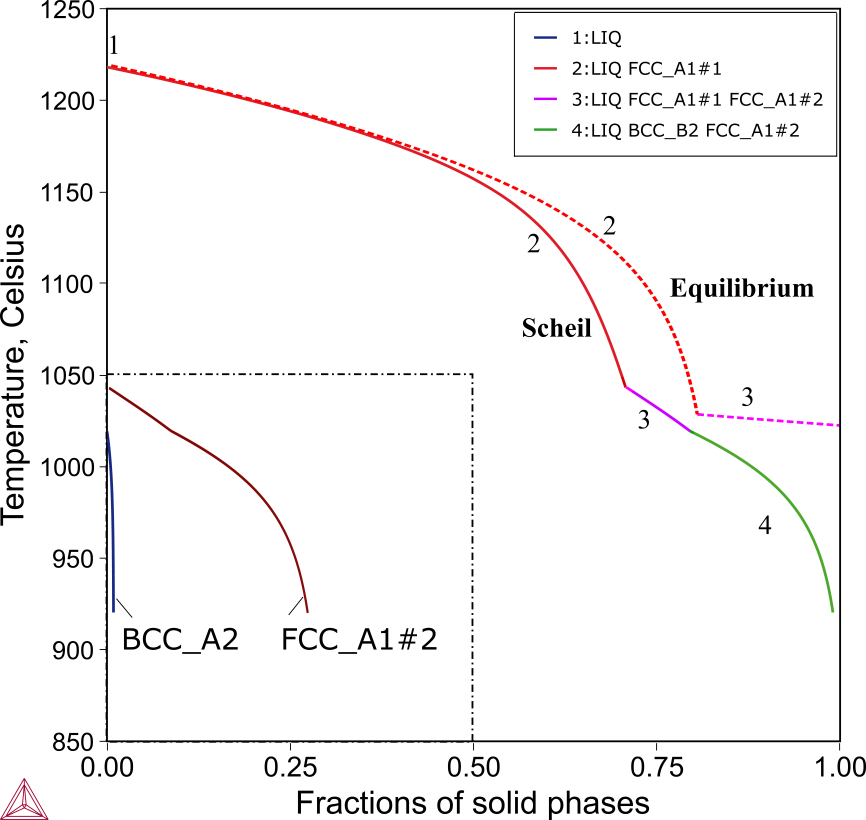
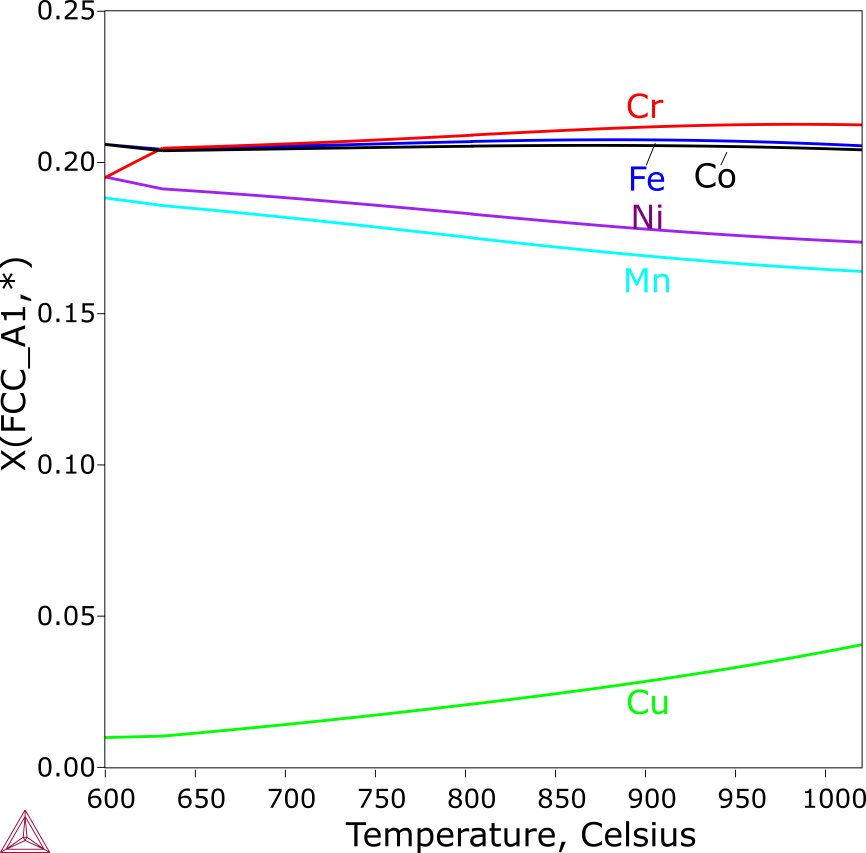
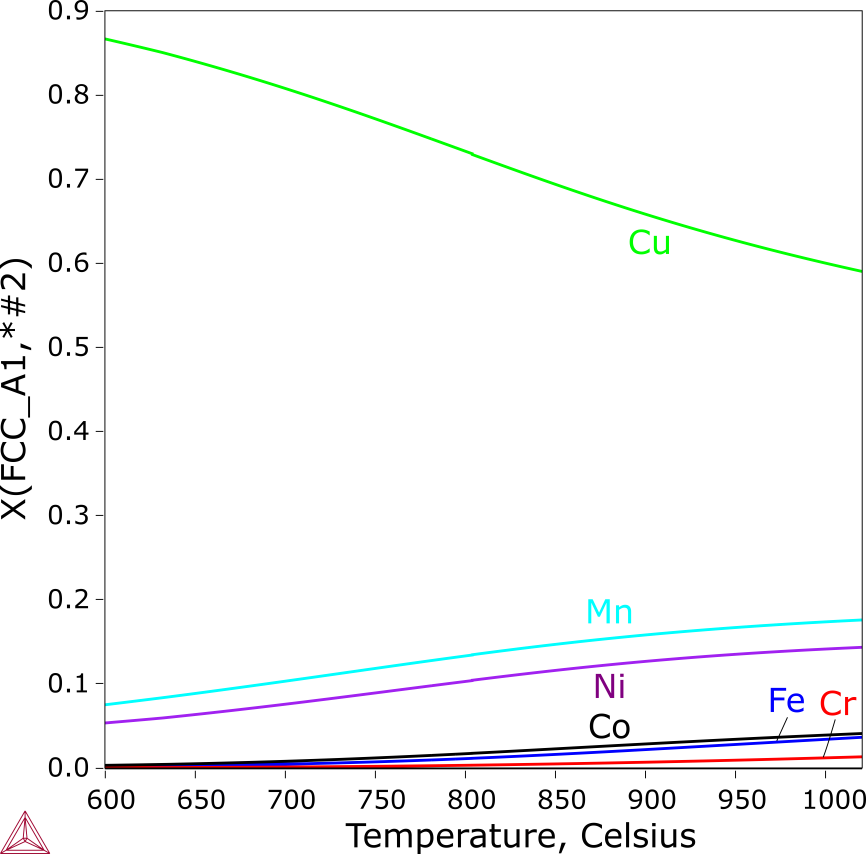
The solidification of the CoCrCuFeMnNi alloy starts with the formation of a Cu-lean FCC_A1 phase, corresponding to the dendrites observed in the as-cast alloy [2004Can]. The second FCC_A1 phase (labeled as FCC_A1#2 in Figure 5), which is Cu-rich starts to form when only about 30% liquid remains. Among the minor alloying elements, Mn and Ni have noticeably higher contents than Co, Cr and Fe. The equilibrium solidification ends at the concurrent formation of the two FCC phases, while the Scheil simulation even predicts the formation of a Cr-rich BCC_A2. No BCC_A2 phase was reported, which probably attributes either its amount was too little to be detected with XRD, or its formation was suspended by the rapid cooling.
Figure 5: Scheil and equilibrium calculations of the CoCrCuFeMnNi alloy. Total and individual solid phase fraction from the Scheil simulation (in solid lines) and equilibrium calculation (in dashed lines) of CoCrCuFeMnNi. The phase fractions of the minor phases (FCC_A1#2 and BCC_A2) are imposed and enclosed with the dashed-dotted lines.
Figure 6: Scheil calculation of the CoCrCuFeMnNi alloy. The composition of the Cu-lean FCC_A1 dendritic phase from the Scheil simulation of CoCrCuFeMnNi.
Figure 7: Scheil calculation of the CoCrCuFeMnNi alloy. The composition of the Cu-rich FCC_A1 interdendritic phase from the Scheil simulation of CoCrCuFeMnNi.
References
[2004Can] B. Cantor, I. T. H. Chang, P. Knight, A. J. B. Vincent, Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A. 375–377, 213–218 (2004).
[2017Mao] H. Mao, H.-L. Chen, Q. Chen, TCHEA1: A Thermodynamic Database Not Limited for “High Entropy” Alloys. J. Phase Equilibria Diffus. 38, 353–368 (2017).
[2017She] S. Sheikh, H. Mao, S. Guo, Predicting solid solubility in CoCrFeNiM x (M = 4d transition metal) high-entropy alloys. J. Appl. Phys. 121, 194903 (2017).
[2018Che] H.-L. Chen, H. Mao, Q. Chen, Database development and Calphad calculations for high entropy alloys: Challenges, strategies, and tips. Mater. Chem. Phys. 210, 279–290 (2018).