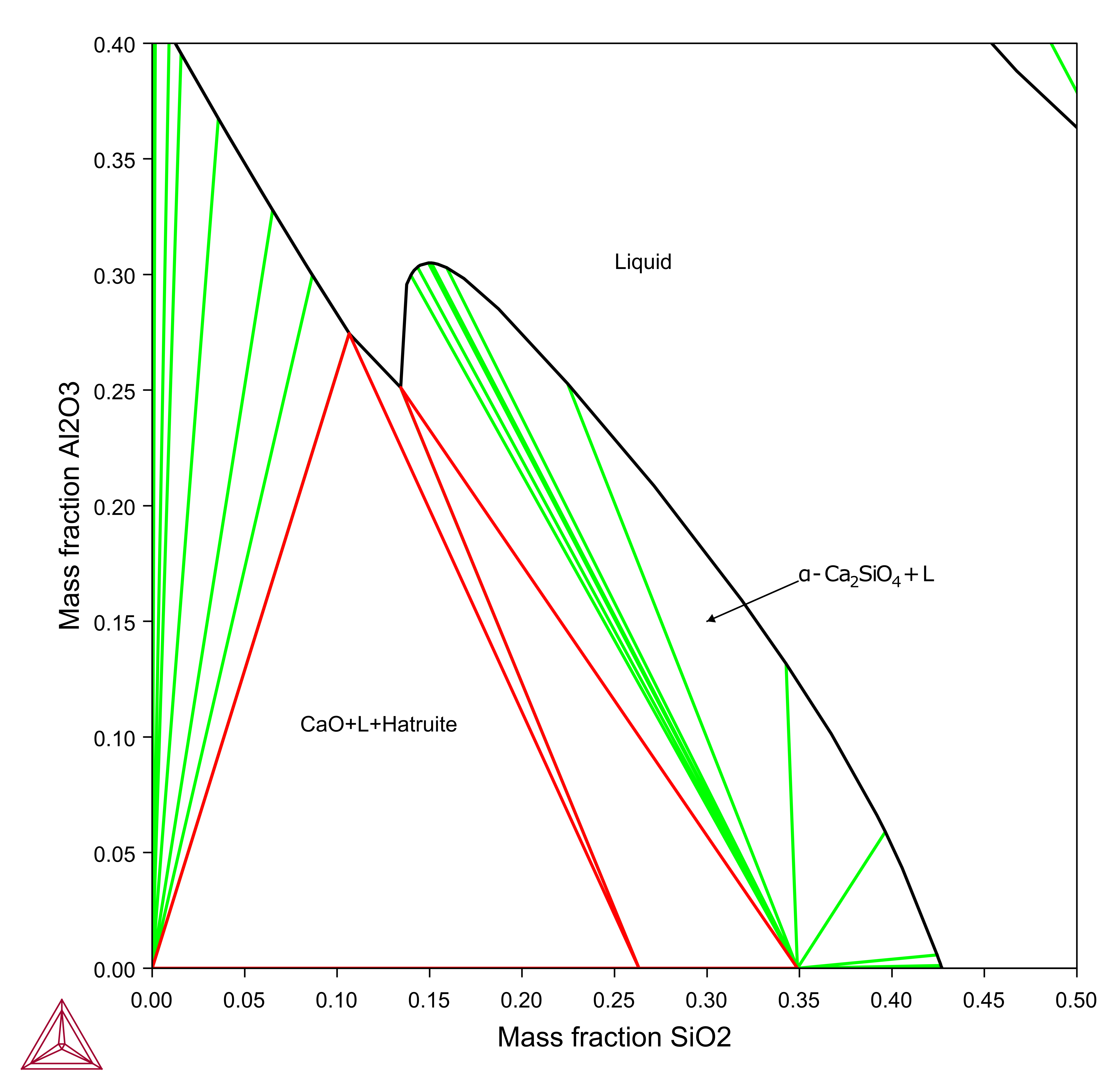
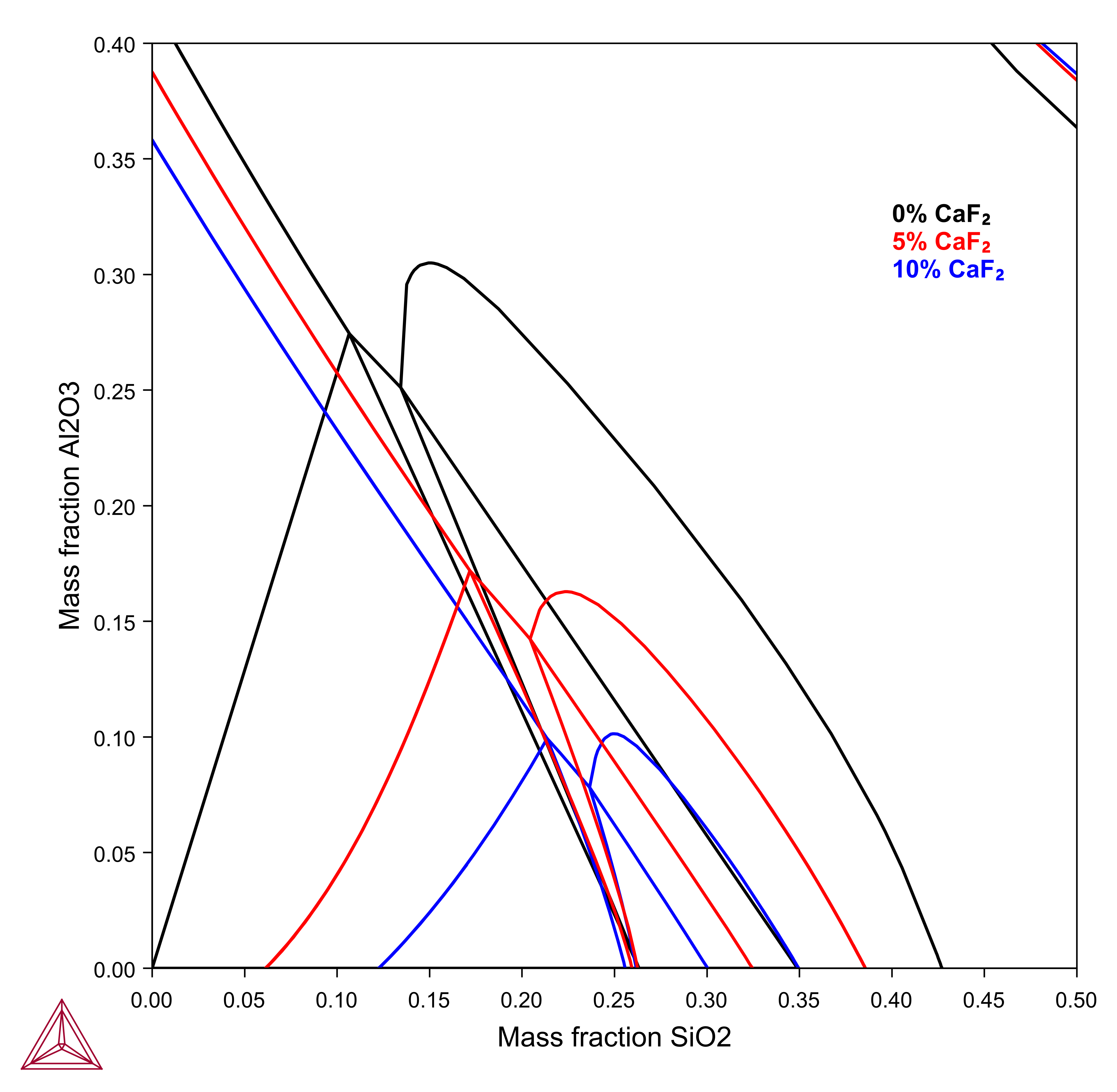
Effect of CaF2 on the Al2O3-CaO-SiO2 Liquidus
Calcium fluoride is known as a powerful reagent in hot metal pretreatment and steelmaking slag, which is based on lime. In recent years, steelmakers were advised to limit the fluorine consumption because of its harmful effects on refractory walls and environmental issues.
The CaO-Al2O3-SiO2 (CAS) system is one of the basic systems for metallurgical slags and has been extensively studied. The effect of CaF2 on the viscosity of slags have been widely investigated and CaF2 is well known to decrease slag melting point and increases lime solubility and slag fluidity as well and improves hot metal refining process, as shown in this example using the TCS Metal Oxide Solutions Database (TCOX).