Fe-Mg-O
Magnesium is of great importance for the steelmaking process. Ladle walls are often made of MgO bricks. Due to its importance in the steelmaking process it is vital to have thermodynamic descriptions containing Mg together with Fe and O, as is shown in this example using the TCS Metal Oxide Solutions Database (TCOX).
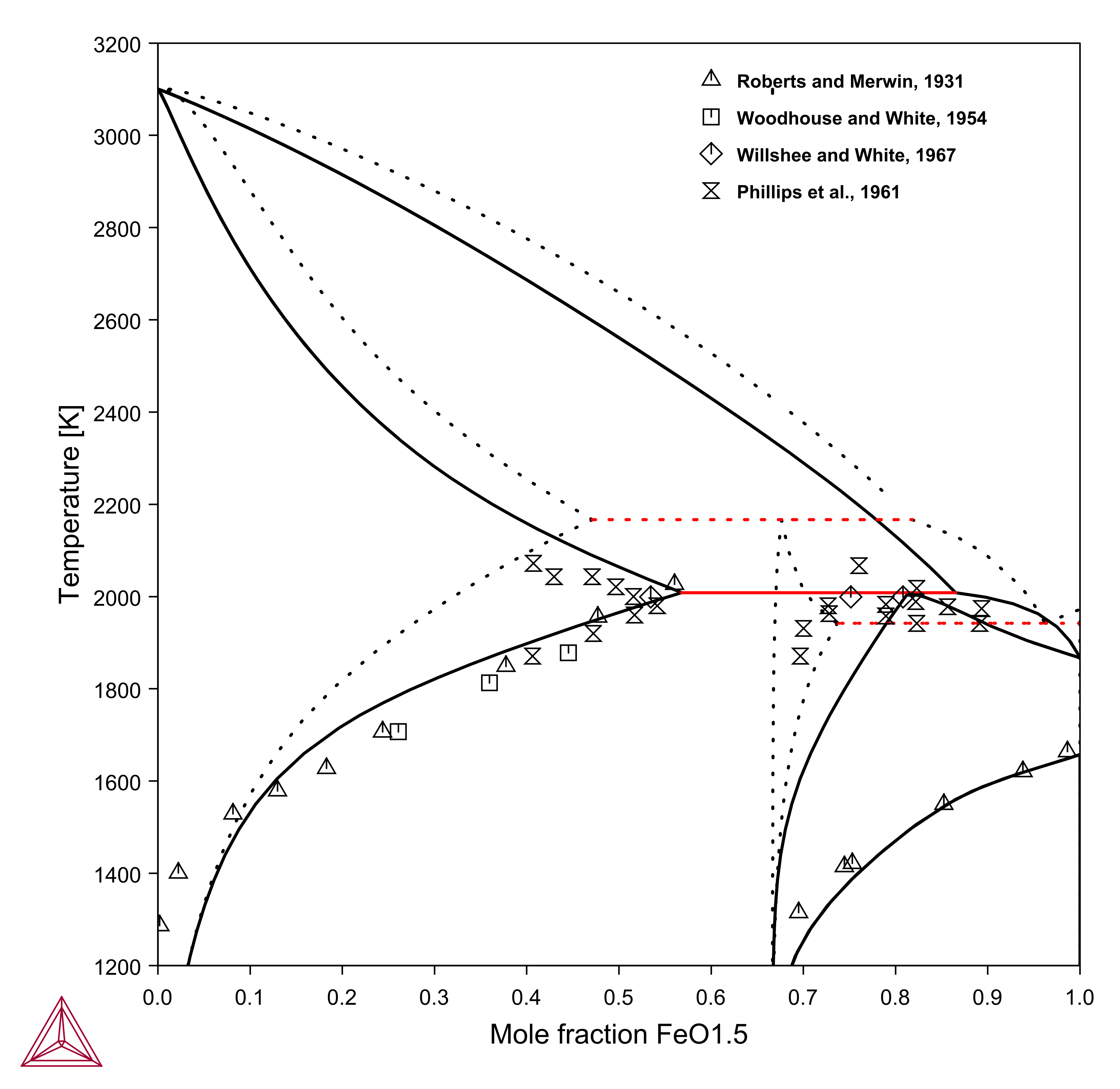
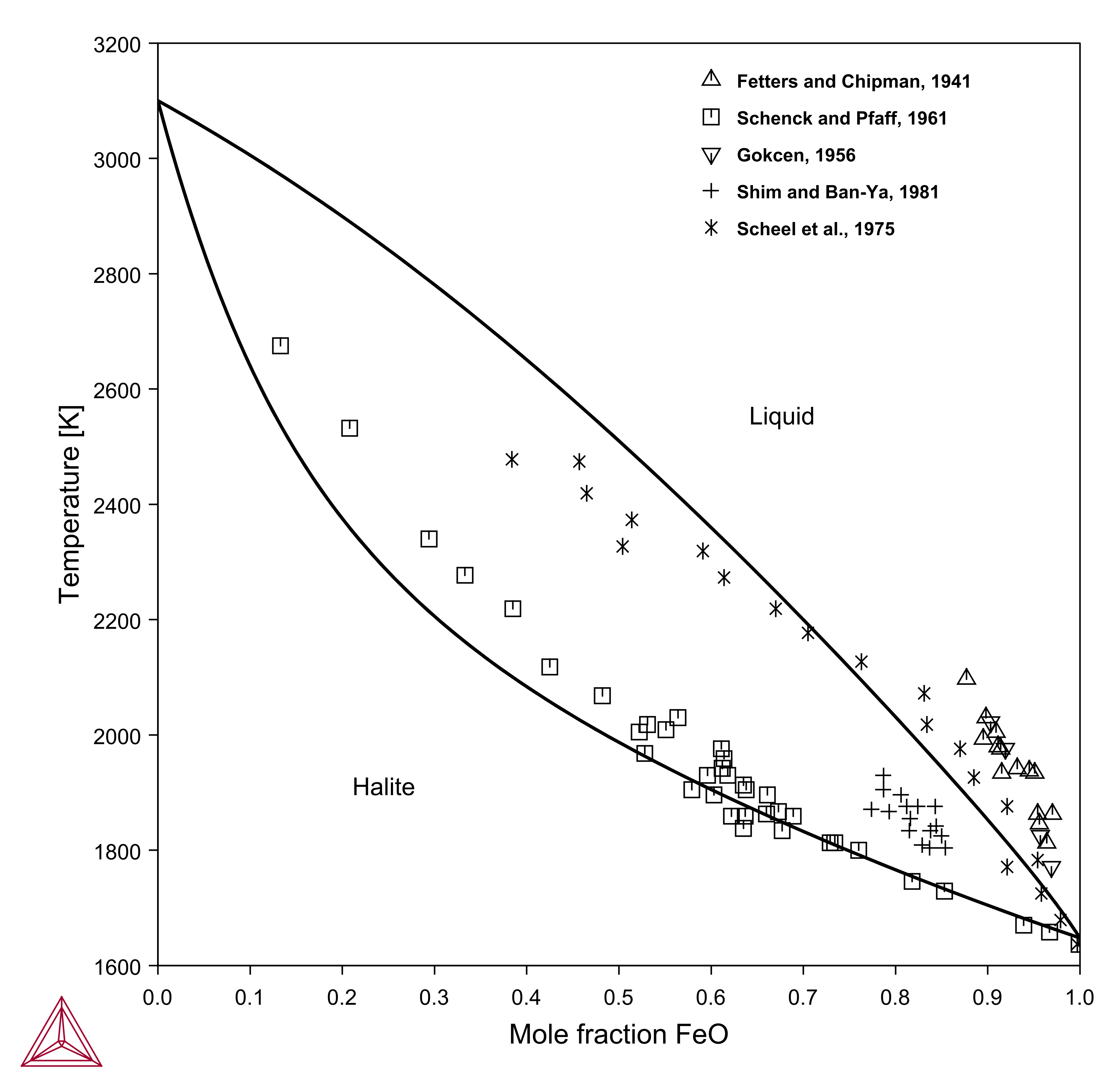
In contrast to Al2O3-MgO, the “missing” condition is really important in systems with multivalent atoms, for example Fe. Experimental phase diagrams are often presented as MgO-FeO or MgO-Fe2O3.
- MgO-FeO: with excess iron, often referred to as iron-saturated section, where the iron oxide consists mainly of FeO. This can be calculated in Thermo‑Calc by using the condition AC(FE,FCC)=1.
- MgO-Fe2O3: Often the phase diagram in air (or oxygen) is presented. This can be calculated in Thermo‑Calc by using the condition AC(O2,GAS)=0.21 (air) or =1 (in oxygen).
Figure 1: Calculated MgO-"Fe2O3" in air. Dashed line is the true binary system MgO-Fe2O3 calculated by setting a very high oxygen potential.
Reference
[2016Dil] D. Dilner, L. Kjellqvist, M. Selleby, Thermodynamic Assessment of the Fe-Ca-S, Fe-Mg-O and Fe-Mg-S Systems. J. Phase Equilibria Diffus. 37, 277–292 (2016).