Viscosity of the Fe-Si-O System
When iron is included in the study of viscosity, it is of great importance to know the atmosphere under which the measurements are made since Fe exists as ferrous (+2) or ferric (+3), or both. The experimental data that reports the partial pressure of oxygen is given a higher weight during the assessment. In Thermo‑Calc:
- when the system is consisted of mainly FeO (often referred to as iron-saturated), you can use the condition of
AC(FE,FCC)=1; - when the system is in equilibrium in air, you can use the condition of
AC(O2,GAS)=0.21(air) orAC(O2,GAS)=1(oxygen).
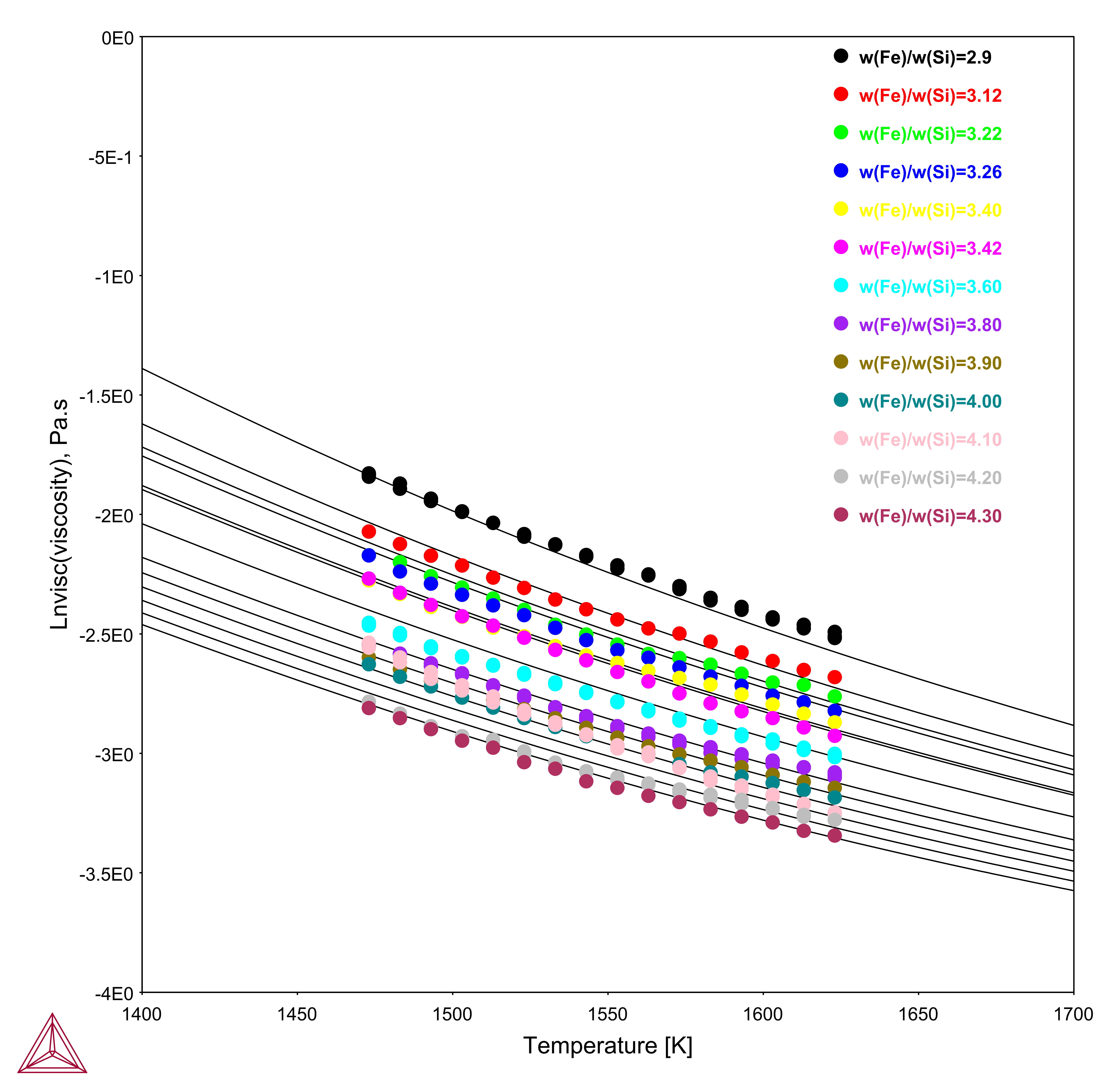
This example, using the TCS Metal Oxide Solutions Database (TCOX), shows how you can calculate the viscosity for the Fe-Si-O system with iron saturation. In the work by Kucharski et al. [1989Kuc], the partial pressure of oxygen was reported to be 6.08e-6 N/m2 that it tends to form Fe+2, and the mass ratio of Fe/Si was measured. Therefore, one can define the components as Fe, Si and O. Since the PO2 was reported, one only needs to define the AC(O2,GAS) instead of AC(FE,FCC).
Reference
[1989Kuc] M. Kucharski, N. M. Stubina, J. M. Toguri, Viscosity Measurements of Molten Fe–O–SiO2 , Fe–O–CaO–SiO2, and Fe–O–MgO–SiO2 Slags. Can. Metall. Q. 28, 7–11 (1989).