Surface Tension of CaF2-CaO and CaO-Al2O3-SiO2
The thermophysical properties available with the TCS Metal Oxide Solutions Database (TCOX) include surface tension.
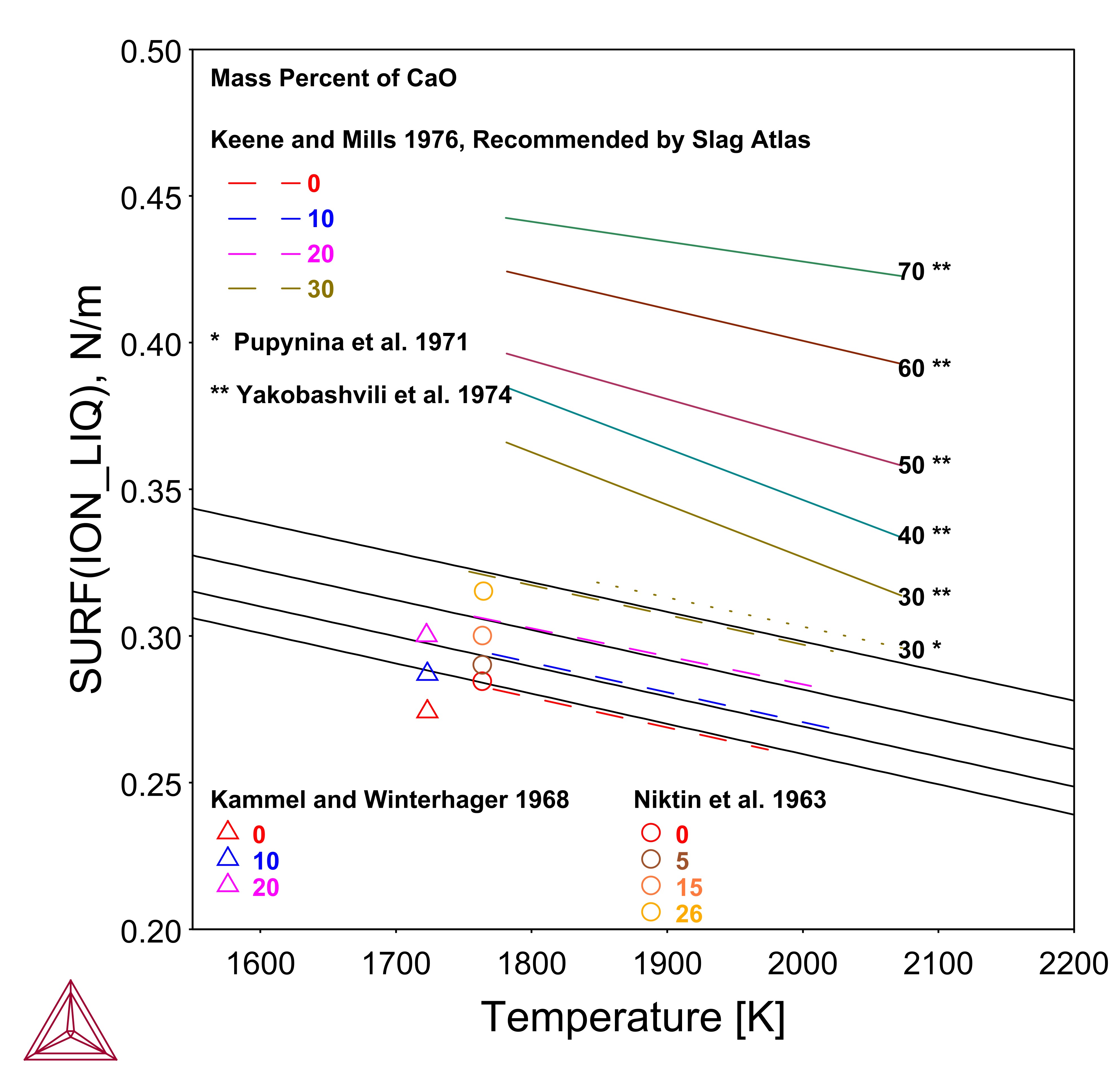
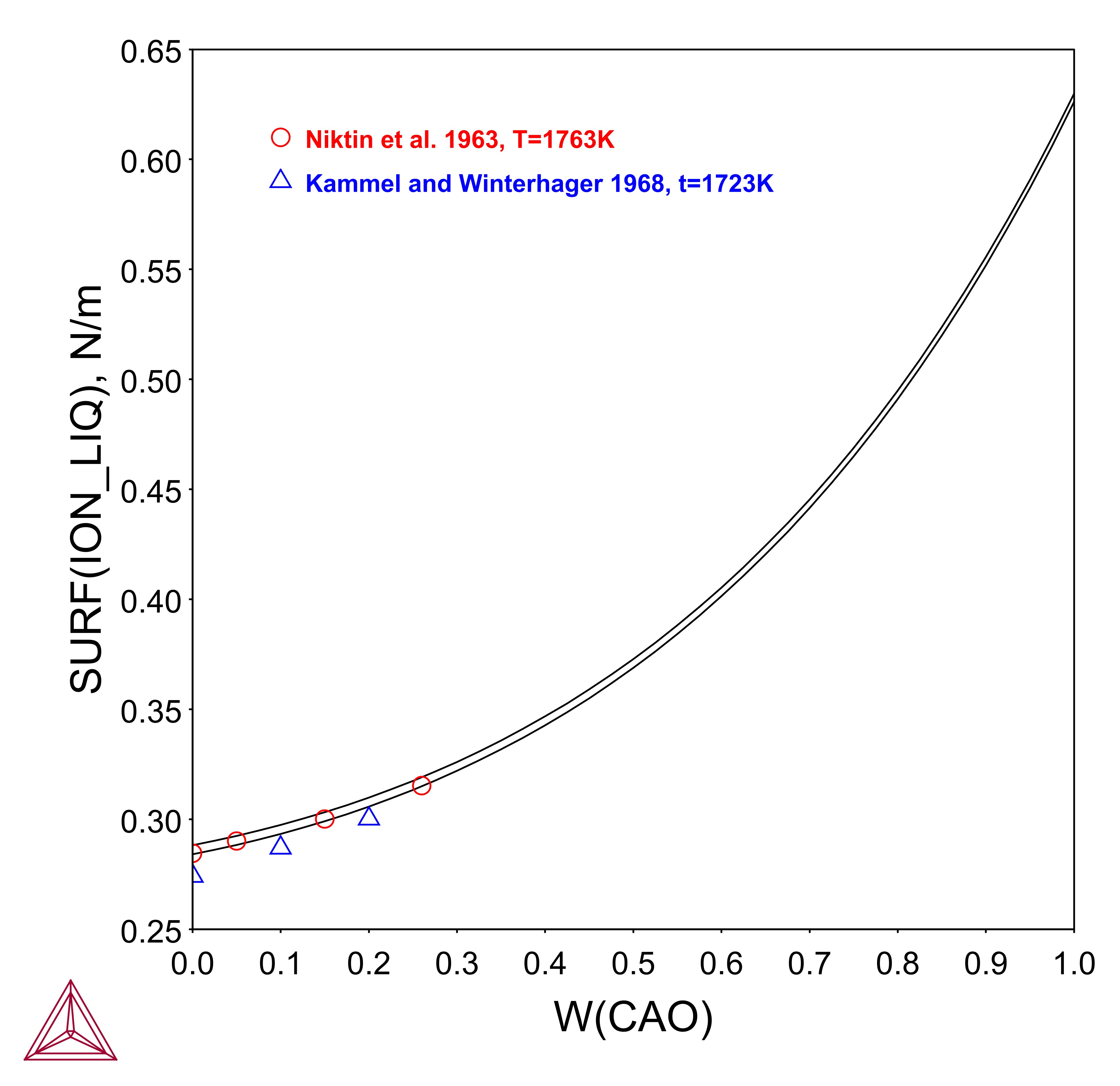
CaF2-CaO
The CaF2 component is well known as network modifier and surface-active constituents in mold flux. With the addition of CaF2 into the slag, it decreases the surface tension.
This example is an outline of the steps to calculate the surface tension of CaF2-CaO using Thermo-Calc and the TCS Metal Oxide Solutions Database (TCOX).
You can define the components of CaF2, CaO, and O as in most industrial applications, and these components are preferred in practice. The gas atmosphere is usually fixed as in air by using P=1E5 and ac(O2,gas)=0.21, but can be changed according to the specific experimental conditions reported. By using the variable of SURF(ION), you can plot the property of surface tension of the ionic liquid phase.
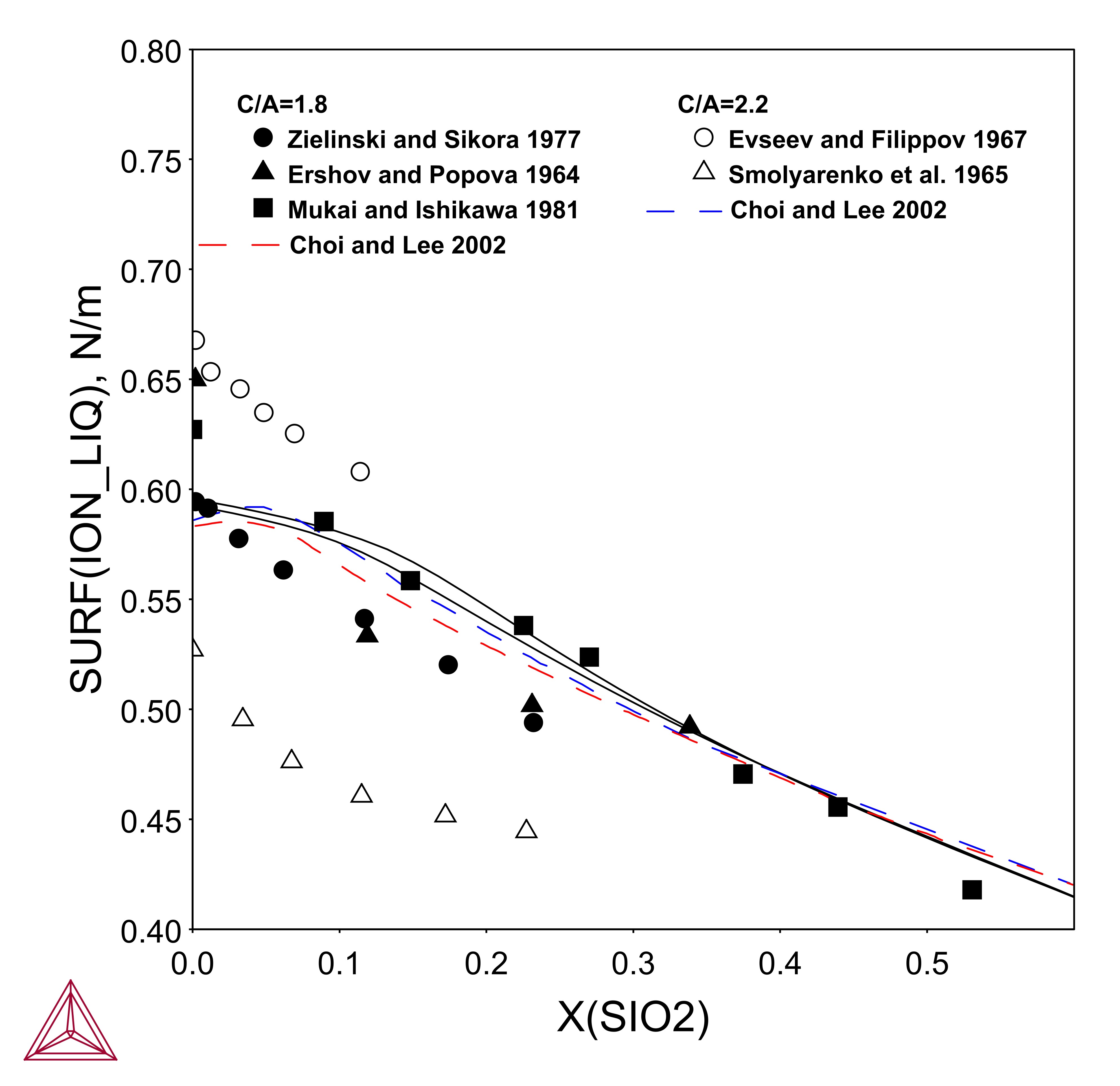
CaO-Al2O3-SiO2
This example using the TCS Metal Oxide Solutions Database (TCOX) demonstrates the comparison between the present calculation and the literature data regarding the surface tension of Al2O3-CaO-SiO2. The calculation is performed by defining the components of Al2O3, CaO, SiO2 and O. After giving the temperature, pressure, size of the system, compositions and oxygen activity, one can calculate the equilibria and then plot the surface tension by using the variable of SURF(ION).
Figure 3: Surface tension of the CaO-Al2O3-SiO2 system with x(CaO)/x(Al2O3)=1.8 and 2.2 at 1873 K.