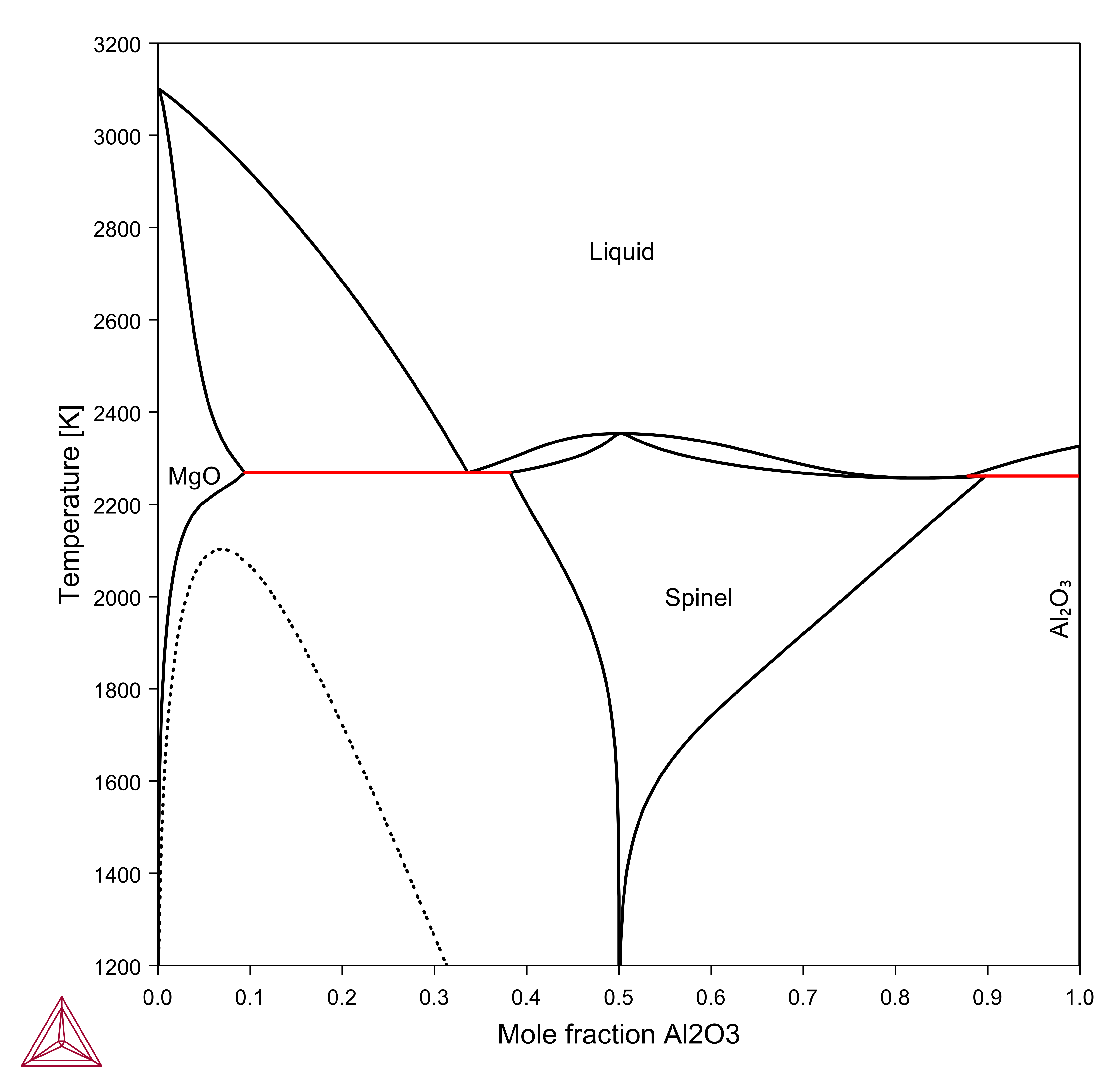
Al2O3-MgO
The system Al2O3-MgO is one of the basic systems to understand metallurgical slags and refractories as is shown in this example using the TCS Metal Oxide Solutions Database (TCOX).
You might want to define the components Al2O3, MgO instead of the elements Al, Mg, O. Since there are three elements, also three components are needed. Al2O3, MgO, and O are chosen here, but other sets are possible and give the same result. When the conditions are set for the Al2O3-MgO “binary”, there is one condition missing after giving temperature, pressure, size of system, and composition, e.g. P=1E5 N=1 T=2000 X(AL2O3)=0.3. One option is to give the oxygen activity as a condition. For systems containing only single valent elements, the absolute value on the activity is not very important.
References
[1992Hal] B. Hallstedt, Thermodynamic Assessment of the System MgO-Al2O3. J. Am. Ceram. Soc. 75, 1497–1507 (1992).
[2004Mao] H. Mao, M. Selleby, B. Sundman, A re-evaluation of the liquid phases in the CaO–Al2O3 and MgO–Al2O3 systems. Calphad. 28, 307–312 (2004).