P_02: Stable and Metastable Carbides - Isothermal
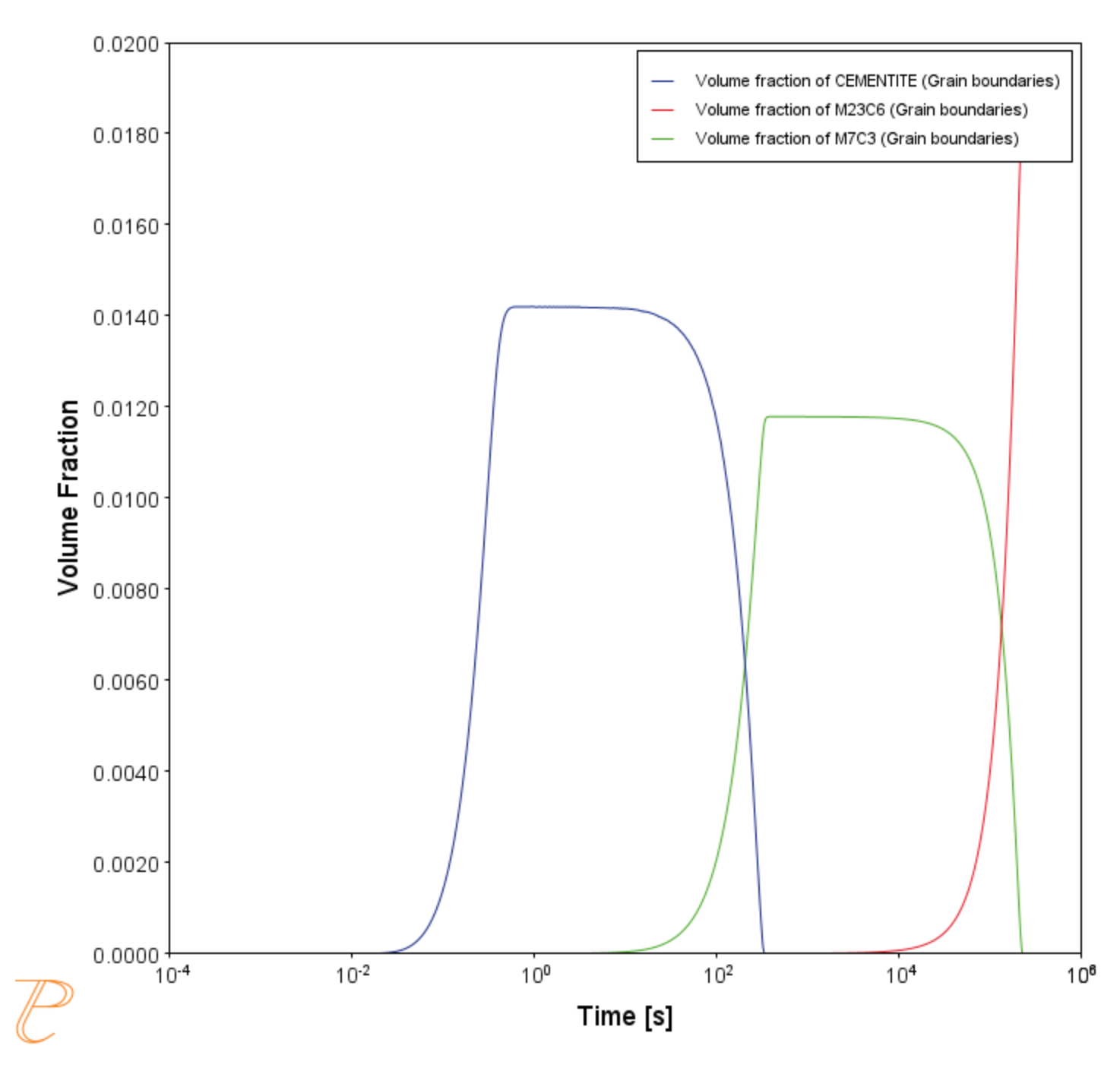
This example simulates the kinetics of precipitation of both stable and metastable carbides from ferrite phase. It demonstrates that metastable carbides (cementite, M7C3) may first emerge and then disappear and the stable phase (M23C6) prevails.
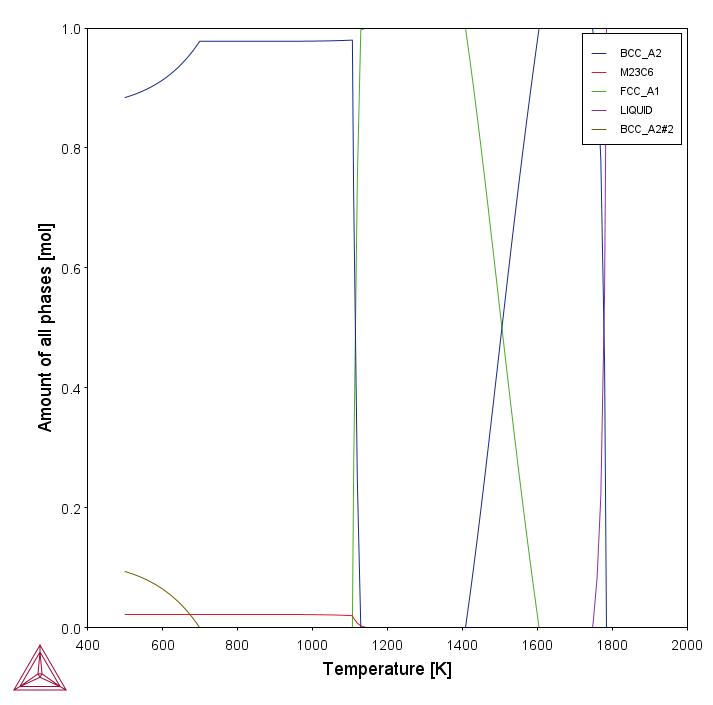
This example uses the Equilibrium Calculator and a one axis calculation to determine how the phases change with temperature. We are interested in the carbide precipitation at 1053 K where only the carbide M23C6 is stable according to the equilibrium calculation. The Precipitation Calculator is used to do an isothermal calculation of the three phases (cementite, M23C6, and M7C3) where cementite and M7C3 are metastable phases.
- Folder: Precipitation Module - TC-PRISMA
- File name:
P_02_Precipitation_Fe-C-Cr_Cementite-M7C3-M23C6.tcu
Example Settings
| System (System Definer) | |
| Database package | Demo: Steels and Fe-alloys (FEDEMO,MFEDEMO) |
| Elements | Fe, C, Cr |
| Conditions (Precipitation Calculator) | |
| Composition | Fe-0.1C-12Cr Mass percent |
| Matrix phase |
BCC_A2 |
| Precipitate phases | Cementite, M23C6 and M7C3 |
| Matrix Phase Data Parameters (Precipitation Calculator) | |
| Grain size (click Show Details to display this setting) | 1.0E-4 m |
| Precipitate Phase Data Parameters (Precipitation Calculator) | |
| Nucleation sites | Grain boundaries |
| Interfacial energy | Cementite 0.167 J/m2, M23C6 0.252 J/m2, M7C3 0.282 J/m2 |
| Calculation Type (Precipitation Calculator) | |
| Calculation type | Isothermal |
| Temperature | 1053 K |
| Simulation time | 400 000 seconds |
Visualizations
Many of our Graphical Mode examples have video tutorials, which you can access in a variety of ways. When in Thermo‑Calc, from the menu select Help → Video Tutorials, or from the main My Project window, click Video Tutorials. Alternately, you can go to the website or our YouTube channel.
Open the example project file to review the node setup on the Project window and the associated settings on the Configuration window for each node. For some types of projects, you can also adjust settings on the Plot Renderer Configuration window to preview results before performing the simulation. Click Perform Tree to generate plots and tables to see the results on the Visualizations window.
There is a variety of information shown in the Visualizations window that can be viewed during configuration and after performing the calculation.
- Thermal Profile: When setting up a calculator on a Configuration window for Isothermal or Non-isothermal Calculation Types, you can preview the profile and adjust settings as needed. When you click a calculator node in the Project window, the matching name of the node is on the tab(s) displayed in the Visualizations window.
For TTT Diagram and CCT Diagram calculations there is nothing shown for the Precipitation Calculator tab in the Visualizations window as there is no Thermal Profile to be defined.
- Plot or Table results: After completing the set up and performing the calculation, to view the matching name of the node on tab(s) in the Visualizations window, either click a Plot Renderer or Table Renderer node in the Project window or click the tabs individually in the Visualizations window.
For this Isothermal example, its Thermal Profile is also displayed in the Visualizations window showing the constant temperature entered for this calculation type. The tab names match the node names in the Project window.
Figure 1: After performing the calculation, you can view the result on the applicable tab. This shows the equilibrium property diagram for the Fe-C-Cr alloy, highlighting what phases are predicted to be at equilibrium for a range of temperatures. The calculation shows that at 1053 K, M23C6 is the stable carbide.
Figure 2: After performing the calculation, you can view the result on the applicable tab. This shows the evolution of the volume fraction of cementite, M7C3, and M23C6 during an isothermal heat treatment of an Fe-C-Cr alloy at 1073 K. The cementite and M7C3 are metastable at this temperature, however their nucleation kinetics are faster than the stable phase, allowing them to form and then dissolve when the next more thermodynamically stable phase nucleates.